The ultimate conditions to get the most out of high-nickel batteries
- The automotive industry has become increasingly interested in the use of high-Ni (nickel) batteries for electric vehicles. However high-Ni cathodes, which make the batteries, are prone to reactivity and instability when exposed to humidity
- Researchers from WMG, University of Warwick have researched the best way to store Ni cathodes to mitigate degradation and improve performance
- Humid or ambient conditions for batteries result in premature capacity fade of the battery, whereas the best condition are dry storage at dew points of around -45oC.
It is common knowledge in battery manufacturing that many cathode materials are moisture sensitive. However, as the popularity of high nickel-based battery components increases, researchers from WMG, University of Warwick have found that the drier the conditions that these cathodes are stored and processed in, then significant improvement in performance of the battery is gained.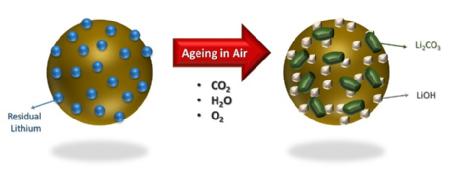
High-Ni (Nickel) batteries are becoming increasingly popular worldwide, with more automotive companies investigating the use of high-Ni batteries for electric vehicles. However, high-Ni cathode materials are prone to reactivity and instability is exposed to humidity, therefore how they are stored in order to offer the best performance is crucial.
In the paper, ‘The effects of Ambient Storage Conditions on the Structural and Electrochemical Properties of NMC-811 Cathodes for Li-ion batteries,’ published in the journal Electrochimica Acta, researchers from WMG, University of Warwick propose the best way to store high-nickel cathodes in order to mitigate premature degradation.
Researchers exposed NMC-811 (high-Ni cathode material) to different temperatures and humidities, then measured the material’s performance and degradation in a battery over a 28 day period, analysing them using a combination of 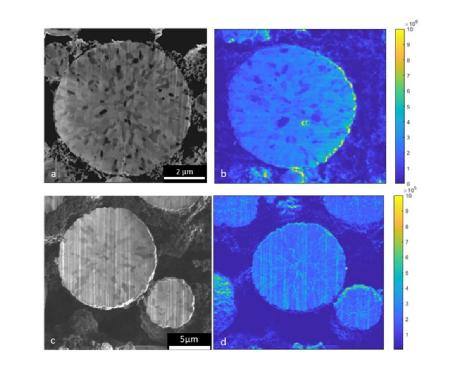 physical, chemical and electrochemical testing. This included high-resolution microscopy to identify the morphological and chemical changes that occurred at the micron and sub-micron scale during the batteries charging and discharging.
physical, chemical and electrochemical testing. This included high-resolution microscopy to identify the morphological and chemical changes that occurred at the micron and sub-micron scale during the batteries charging and discharging.
The storage conditions included vacuum oven-dried, as exposed (to humidity) and a control measure. Researchers looked for surface impurities, which include carbonates and H2O, and found there were three processes that can be responsible for impurities, including:
1. Residual impurities emanating from unreacted precursors during synthesis
2. Higher equilibrium coverage of surface carbonates/hydroxides (present to stabilise the surface of Ni-rich materials after the synthesis process)
3. Impurities formed during ambient storage time
They found that in all conditions, (oven dried and as-exposed) showed inferior first discharged specific capacity and cycling performance, compared to the control. However the as-exposed measure showed that after 28 days of ambient moisture exposure the H2O and CO2 react with the Li+ ions in the battery cell, resulting in the formation of lithium carbonate and hydroxide species.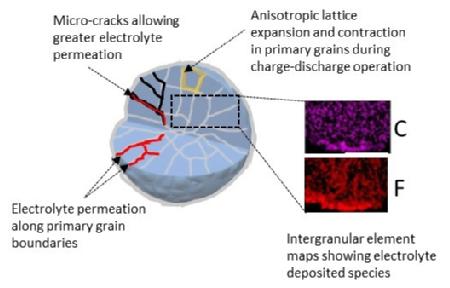
The formation of carbonates and oxides on the surface of NMC-811 contribute to the loss of the electrochemical performance during ageing of the materials, due to the inferior ionic and electronic conductivity, as well as the electrical isolation of the active particles. This means that they can no longer reversibly store lithium ions to convey “charge”. SEM analysis confirmed the inter-granular porosity and micro-cracks on these aggregate particles, following the 28 days of ambient exposure.
They can therefore conclude that the driest conditions, at dew points of around -45 oC, are the best for storing AND processing the materials, in order to then produce the best battery performance. Humidity conditions and exposure at junctions along the manufacturing process will cause the materials and components to experience; this results in shorter battery lifespan.
Dr Mel Loveridge from WMG at the University of Warwick comments:
“Whilst moisture is well known to be problematic here, we set about to determine the optimal storage conditions that are required to mitigate unwanted, premature degradation in battery performance. Such measures are critical to improve processing capability, and ultimately maintain performance levels. This is also of relevance to other Ni-rich systems e.g. NCA materials.”
Professor Louis Piper from WMG at the University of Warwick adds:
“Considerable global research effort will continue to focus on these materials, including how to protect their surfaces to eliminate risks of parasitic reactions prior to incorporation into electrodes. In the UK, leading research by the Faraday Institution has a project consortium entirely devoted to unravelling the degradation mechanisms of such industry-relevant materials.”
ENDS
18 NOVEMBER 2020
NOTES TO EDITORS
High-res images available at:
https://warwick.ac.uk/services/communications/medialibrary/images/october_2020/the_effects_of_ambient_air_storage_on_the_surface_stability_of_nmc-811.jpg
(1) Caption: The effects of ambient air storage on the surface of NMC-811
Credit: WMG, University of Warwick
https://warwick.ac.uk/services/communications/medialibrary/images/october_2020/post-mortem_nmc811_particle.jpg
(2) Caption: a-b) Post-mortem NMC811 particle, with no prior exposure to moist air, analysed by FIB-SIMS, targeting Lithium detection. c-d) Post-mortem NMC811 particle, after 28 days exposure to moist air, analysed by FIB-SIMS, targeting Lithium detection.
Credit: WMG, University of Warwick
https://warwick.ac.uk/services/communications/medialibrary/images/october_2020/schematic.jpg
(3) Caption: Schematic illustration of particle breakdown during charge-discharge of a battery
Credit: WMG, University of Warwick
Paper available to view at: https://www.sciencedirect.com/science/article/pii/S0013468620317515?via%3Dihub
FOR FURTHER INFORMATION CONTACT:
Alice Scott
Media Relations Manager – Science
University of Warwick
Tel: +44 (0) 7920 531 221
E-mail: alice.j.scott@warwick.ac.uk
FOR FURTHER INFORMATION CONTACT:
Alice Scott
Media Relations Manager – Science
University of Warwick
Tel: +44 (0) 7920 531 221
E-mail: alice.j.scott@warwick.ac.uk
