ProBe: Progenitors of Beta Cells in Pregnancy
Functional beta-cell mass (FBM) doubles in a few weeks of mouse pregnancy. The contribution of different sources to FBM expansion in pregnancy is still unclear. Our earlier published work (S. Abouna, et al., Organogenesis, vol. 6, pp. 125-33, 2010) demonstrates that during pregnancy a significant number of new beta-cells arise from non-beta-cell precursors, the remainder coming from replication of existing beta-cells. The aim of this project is to clarify the identity and role of precursor cells (of which there could be more than one cell type). We have employed Pulse-Chase Lineage Tracing (PCLT) in two different experiments, using mice with transgenes CreERT to label duct and beta cells respectively. Analytical power is enhanced by coupling PCLT with a new microscopy platform, Toponome Image System (TIS), that can co-locate 40 or more different proteins in the same pixel of the same intact tissue section. The progeny of labelled cells are marked, and tracked from time point to time point, with the percentage contribution to beta-cell neogenesis unambiguously attributed. Imaging of the lineage label alongside detailed cell morphology, plus molecular co-expression patterns, formed using an additional 30 differentiation markers, can reveal the differentiation route for formation of new beta-cells. We will develop novel analytical tools based on mutual information, maximal information coefficient (MIC), nonlinear manifold learning and deep learning. Tracking of signatures from molecular co-expression of protein markers should reveal ancestors of beta-cells. This new knowledge will advance understanding of beta-cell formation and demonstrate the role of non-beta-cells in FBM expansion during pregnancy.
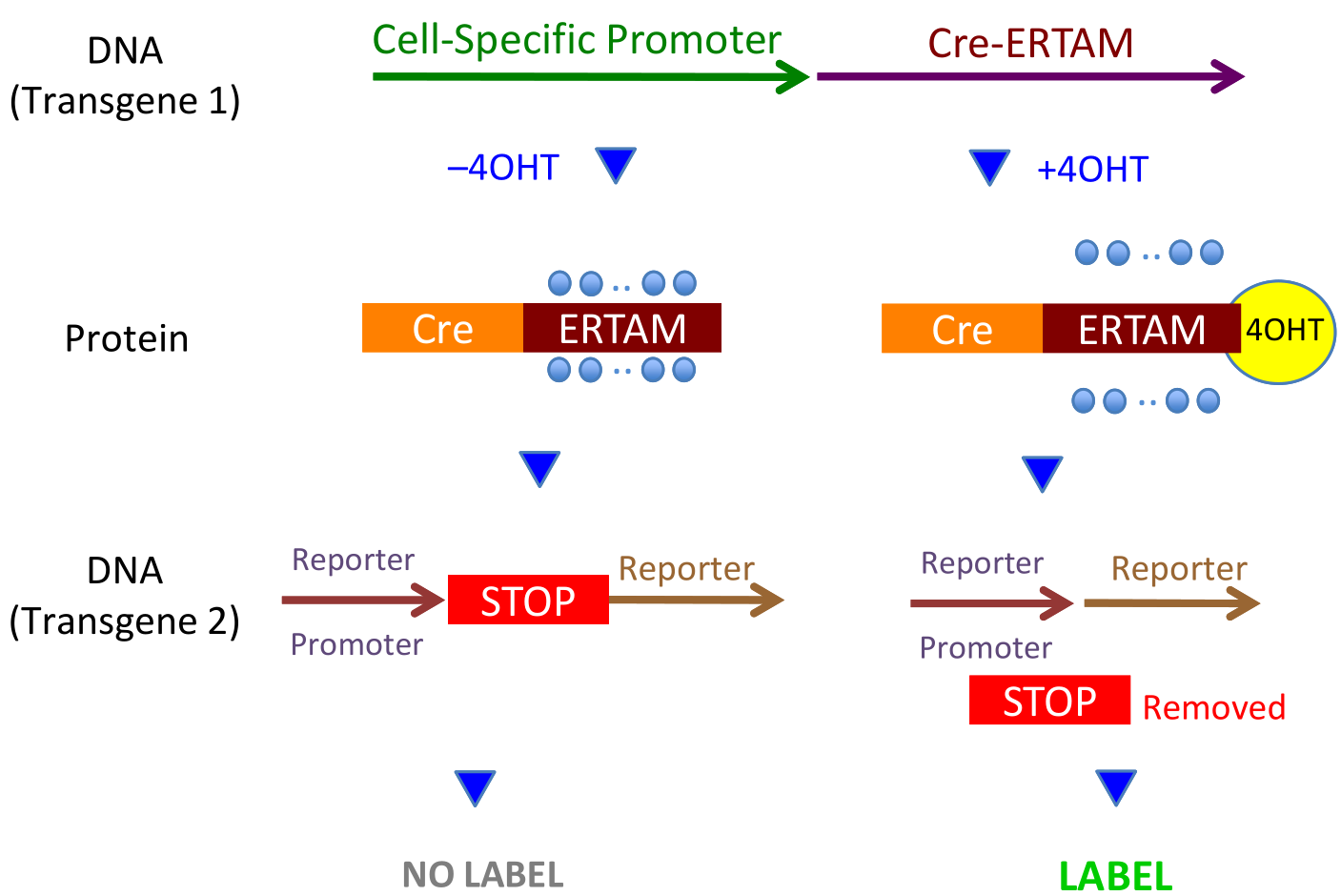
without Tamoxifen (or it’s metabolite, 4OHT), transgene1 (Line 1) produces (Line 2, left) the CreERT fusion protein which stays inactive in the cytoplasm. With 4OHT (Line 2, right), the blocking heat shock proteins (blue disks) are displace, CreERT becomes active, enters the nucleus, and snips out the floxed STOP codon. Thus, without 4OHT, the cell is not labelled, and, with 4OHT, it is labelled.
Lay Summary of the Project
During normal human pregnancy, the mother's need for insulin is doubled. To fulfil this need, her body creates additional insulin-producing cells--these are called beta cells. This project's question is: Where do the additional cells come from in normal pregnancy? Understanding the body's production of new beta cell is an important biological problem in its own right. This understanding is also important for the treatment of diabetes, because a deep understanding of diseased physiology rests on understanding normal physiology. Experiments cannot be done on human subjects. We use mice, whose physiological response to pregnancy is fortunately similar to that of humans. For example, during pregnancy, the mass of beta cells more than doubles in mice, and the same is true for humans. This remarkable increase takes place over only a few days in a mouse, which is convenient for our study. This project's question can be recast: During pregnancy, which types of cells transform themselves into beta cells? Until recently, the accepted wisdom on this question was provided by a wonderful experiment carried out by Melton and Dor. They start with transgenic mice, that is, mice whose DNA has been altered in a certain way. This alteration has little effect on the physiology of the mouse until the mouse is injected with a certain hormone (tamoxifen), at which point the altered DNA starts to produce, but only in one particular type of cell--in the Dor-Melton case, only in beta cells--a certain protein (HPAP) visible under the microscope after staining. The cell is said to be "labelled by HPAP". What makes the experiment so informative is that, when an HPAP-labelled cell divides (replicates), then the cells resulting from division are also labelled. A labelled cell must have had labelled ancestors, and an unlabelled cell must have had unlabelled ancestors. This gives a mechanism for tracking the ancestry of a cell, which works whether or not the cell changes to a cell of different type. Melton and Dor showed that, under circumstances that do not occur in nature (surgical removal of part of the pancreas), new beta cells come only from existing beta cells, via the usual process of cell division. Our group recently repeated the Melton-Dor experiment using normal pregnancy to create a large but normal demand for additional insulin. Under these conditions, the same labelling technique leads to a very different conclusion--a substantial proportion of the new beta cells do NOT arise through replication of existing beta cells, but come from somewhere else. Our experiment was not designed to show where the new beta cells did come from, even though we could be sure that some came from division of existing beta cells. Our objective now is to determine with precision the various types of ancestors of new beta cells in pregnancy. This would be a major step towards developing alternative sources of beta cells for transplantation or to develop new strategies to maintain/restore the endogenous pancreatic beta cell mass. The University of Warwick’s robotically controlled TIS microscope, is one of only two in the world of this type (the other is at the Hershey Campus of Penn State University). In Germany there are several older versions of this machine, working on the same principle, but with fewer refinements. The machine allows us to deduce, from the expression of different proteins at different time points, how a cell changes while becoming a beta cell. TIS can detect 40 or more proteins at the same place in the same tissue section, instead of the 2 or 3 detectable through conventional microscopy. This great power of TIS will enable us to identify which of many suggestions are correct as to the identity of ancestors of beta cells.
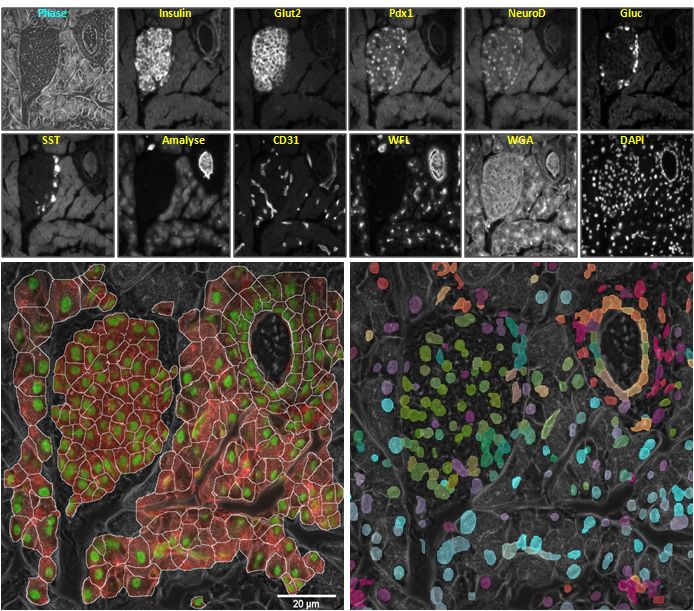
Phase image and fluorescence images of the same pancreatic tissue specimen using eleven different tags; Bottom row, each overlaid on the phase image: Left: preliminary results of cell segmentation and Right: colourised cells with scaled values of first 3 nonlinear embedding coordinates used as RGB values. Different types of cells within the islet area (e.g., β-cells, α-cells, δ-cells) and heterogeneous ductal cells can be seen colourised with different colours in the lower right image. There is a scale bar in the lower left image and in no other because all 14 images display exactly the same pixels but each time looking at a different protein or with a different technique. A careful look at the cell segmentation displays some ‘cells’ with two nuclei. It is possible to have two nuclei in one cell during mitosis, a rare event for whose detection we have recently developed algorithms.
The PrIDE Software
As part of the ProBe project, we have developed the PrIDE (ProBe Image Data Explorer) software which is able to load, manipulate, visualise, and analyse the image data generated in this project. A PDF detailing the software features can be downloaded by clicking here.
Project Personnel
Dr Stella Pelengaris
Dr Linda Cheung




