Atom Transfer Radical Cyclisation
Transition metal catalysed atom transfer radical cyclisation (ATRC)1 and polymerisation (ATRP)2 reactions have been extensively studied over the last few years. The driving force for this research has been the desire to find non-reductive catalytic alternatives to organotin hydrides in mediating radical cyclisation reactions in organic synthesis, and the need to prepare living polymers with a high degree of control for novel materials applications.
We have introduced a range of new ligands for these processes (see Fig. 1).
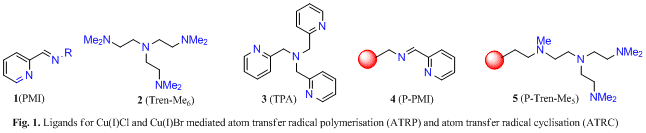
Polymerisation (ATRP): Between 1994-2000 in collaboration with Prof. D. Haddleton we developed copper mediated atom transfer radical polymerisation of methacrylates and styrenes using pyridylmethanimine ligands 1 (5 joint papers,5-9 funded by the EPSRC, GR/L10314: a-5. rated).
Cyclisation (ATRC): Copper mediated atom transfer radical chemistry has recently been developed to overcome the problems associated with utilising toxic organotin hydrides in mediating radical cyclisation reactions. In this approach Cu(I) is used to abstract a halogen from a substrate (e.g. 6) to generate the radical 8. In this process the metal increases oxidation state, scheme 1. After cyclisation the metal then donates a halogen back to the cyclised radical 9 thus regenerating the active Cu(I) catalyst and retaining functionality in the product. In early studies this was low yielding, required high temperatures and only worked with activated precursors (e.g. trihalo derivatives such as 6).10 Better results were obtained when ligands for copper (e.g. bipyridine) were used in the reaction.11
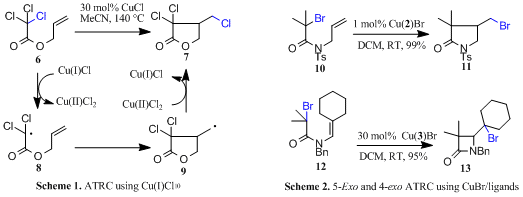
Development of efficient catalysts: In a fully funded PhD collaboration with Knoll Pharmaceuticals we probed the effect of ligand structure on 5-exo-trig,12 6-exo-trig13 and 8-12 endo-trig cyclisations13 to give nitrogen heterocycles. We discovered that ATRC using copper bromide and ligands 1-2 allows efficient reactions to take place at room temperature utilising only simple monohalo precursors (e.g. 10) with termination via bromine transfer, Scheme 2.12-14 Structure activity relationships of the catalysts were probed by kinetic investigations.15
β-Lactam synthesis and additions to alkynes: We next expanded the scope of the reaction with an Industrial CASE collaboration with Aventis by applying this chemistry to the preparation of b-lactams via 4-exo-trig cyclisation, e.g 12.16 While the majority of previously published syntheses of b-lactams mediated via radical reactions proceeded with poor yields it was possible to prepare a range of derivatives (e.g. 13) in high yield (<95%). However, only ligand 3 was successful in mediating these 4-exo cyclisations. Cyclisation onto alkynes (e.g. 14) were also studied. In these processes the product outcome was determined by the type of ligand utilised, Scheme 3.17 Thus, cyclisation of 14 by ligand 1 furnished 15 (X = Br) as expected but a different product terminated by a hydrogen atom 15 (X = H) was produced if ligand 2 was used, Scheme 3.
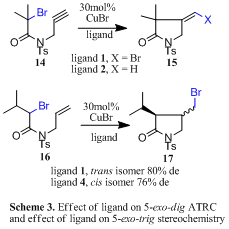
Solid supported chemistry: In an Industrial CASE collaboration with AstraZeneca we developed a range of solid supported catalysts 4-5 which successfully mediated 4-exo, 5-exo, and 6-exo ATRC’s as well as 5-endo radical-polar crossover reactions.18,19 Kinetic profiles and re-usability data for the catalyst systems were studied. Interestingly, the stereochemical outcome of the cyclisation of 16 was dependant upon whether the reaction was homogenously (ligand 1) or heterogeneously (ligand 4) catalysed, Scheme 3. In other work we investigated the cyclisation onto dienes and trienes.20
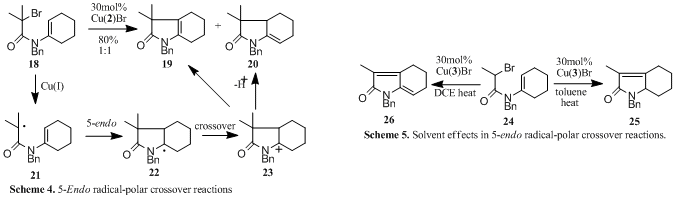
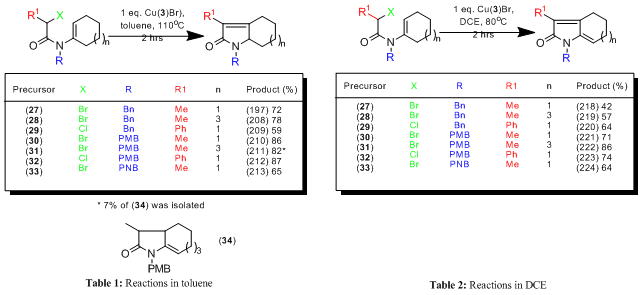
Radical-polar crossover reactions: In collaboration with Lilly Research we developed copper21 and cerium22 mediated 5-endo radical-polar crossover reactions of enamides. Thus reaction of 18 with Cu(2)Br furnishes a 1:1 mixture of the alkene regioisomers 19 and 20 arising from cyclisation of radical 21, oxidation of the cyclised radical 22 to the acyliminium ion 23 in a crossover process, followed by elimination of a proton, Scheme 4. The effect of different copper catalysts was probed and remarkable solvent effects determined. These reactions were found to be highly solvent dependant giving rise to monoenes (e.g. 25) or dienes (e.g. 26) depending upon the reaction conditions. Thus cyclisation of 24 furnishes the alkene 25 using Cu(3)Br in toluene but produces the diene 26 when the same catalyst is used in DCE, Scheme 5 (see also Tables 1-2).23 In recent unpublished work we have demonstrated that 4-exo, 5-exo and 5-endo trig cyclisations are efficiently mediated in water and ionic liquids.20
22) A.J. Clark, C.P. Dell, J.V. Geden, P. Mawdsley, J.P. McDonagh, Org. Lett., 2003, 5, 2063.23) A.J. Clark, C.P. Dell, J.P. McDonagh, C. R. Acad. Sci. Ser IIc: Chim, 2001, 4, 575
