New Technologies for EPR/DNP-NMR Spectro-Electrochemistry
Paramagnetic species are ubiquitous as intermediates or products of electrochemical processes.
Consequently Electron Paramagnetic Resonance (EPR) has long been recognised as a powerful
technique for the in-situ spectroscopic characterisation of electrode reactions. However, in general,
in-situ electrochemical cell performance has been sacrificed (e.g. detrimental effects on the
electrochemical time constant, integrity of the voltammetric signal, etc.) to maximise EPR S:N, and
the advances in modern EPR instrumentation (e.g. high-sensitivity bridges, novel resonators, high
fields, pulsed EPR, etc.) have not been exploited. Nuclear Magnetic Resonance (NMR), even though
it has the ability to provide electronic, structural and dynamical information, has been less
frequently used, primarily because of low intrinsic sensitivity. For NMR to be more widely exploited
in the study of electrochemical reactions it is necessary to considerably increase the sensitivity;
Dynamic Nuclear Polarisation (DNP), a hybrid of EPR and NMR should provide the required
sensitivity gain and particularly where one can use the electrochemically generated paramagnetic
species as the DNP polarisation sources.
With the exceptional detection sensitivity of modern EPR instrumentation, and unprecedented
sensitivity gains in NMR using DNP, we have a unique opportunity to radically re-think the approach
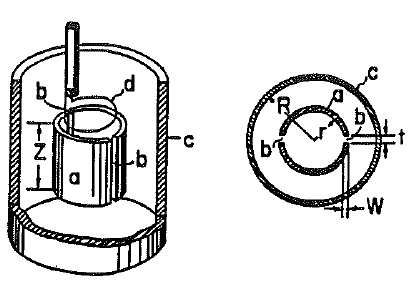
to in-situ electrochemical EPR and DNP-NMR spectroscopy. Various micro-scale electrochemical-
EPR/DNP-NMR cells, with small time constants, will be developed allowing the characterisation of
transient species in electrochemical systems with lifetimes many orders of magnitude shorter than
previously attainable. The optimal approach to spectroscopic observation of short-lived species will
be via electrochemical potential modulation of the magnetic resonance signal, with eventual lock-in
detection at the modulation frequency. The micro-scale electrochemical cell, combined with high
magnetic resonance sensitivity will facilitate this approach. The technology to be developed will thus
provide major new insights into electrode and interfacial reactions.
The project will draw on the leading expertise of the Warwick Electrochemistry and Interfaces Group
(Professors Unwin and MacPherson) in the design (using finite element modelling), development
and application of novel micro-scale in-situ electrochemical techniques. A wide range of systems will
be open for study, including the simple reductive or oxidative generation of radical anions or cations,
respectively, for which many possibilities exist.