Kelvin Goh
Introduction:
I am a physics PhD student based in the University of Nottingham. My PhD research is on the investigation of quantum molecular dynamics of dihydrogen endofullerenes using Nuclear Magnetic Resonance (NMR) and Inelastic Neutron Scattering (INS) spectroscopy.
Research:
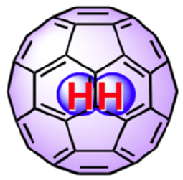
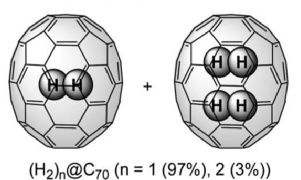
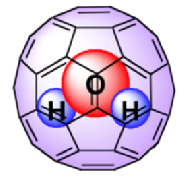
In recent years, remarkable advances in synthetic chemistry have enabled the the permanent trapping of small molecules inside nanoscopic spherical fullerenes. The technique used to encapsulate small molecules inside the Buckyballs is known as "molecular surgery", as it involves systematic process of opening an orifice, inserting the molecule and closing the orifice of the fullerene cages. [1, 2] This technique was first introduced by Komatsu et al at Kyoto University to produce the first molecular endofullerenes, which is the H2@C60. The success of this synthesis gave birth to a new collaboration of scientists of various disciplines from all over the world dedicated to studying these phenomenal complexes. This results in the synthesis of other variation of endofullerenes such as H2O@C60 and H2@C70 in recent years. [3]
Endofullerenes possess many unique properties. Highly unstable atoms or molecules such as Nitrogen atom and H2 are able to remain stable while trapped inside the cage. This is because atoms or molecules trapped inside the cage do not interact chemically with the inert interior of the fullerene cage as well as with other atoms and molecules outside the cage. Endofullerenes uniquely enable trapped molecules to be studied in isolation while arranged in a lattice structure, where they are free to rotate and translate within the cage without any form of chemical bonding. When the entrapped molecule is H2 the inter-fullerene interactions are virtually negligible, revealing themselves only at the very lowest temperatures. The dynamics of the trapped molecule within the cage is highly quantum mechanical. They behave as molecular quantum rotors and exhibit quantization of all motional degrees of freedom, such as vibrations, rotations, and translations. Dihydrogen trapped molecules such as H2 and H2O exhibit nuclear spin isomerism which restricts the dynamics of certain spin species of dihydrogen molecules to certain rotational states.
My interest in these molecules lies in the quantum dynamics of the molecules trapped inside the fullerene cages. This includes the quantum rotation of the trapped molecule, inter-fullerene interactions and the nuclear spin conversion between the spin isomers. The experimental techniques used to study the endofullerenes include low-temperature field-cycling relaxometry, mili-Kelvin NMR spectroscopy and inelastic neutron scattering.
References:
1. Rubin Y (1999) Ring opening reactions of fullerenes: Designed approaches to endohedral metal complexes. Top Curr Chem 199:67–91.
2. K. Komatsu, M. Murata, and Y. Murata. (2005) Science, 307:238-240.
3. Kurotobi K, Murata Y (2011) A single molecule of water encapsulated in fullerene C60 Science 333:613–616.
Supervisors:
1. Anthony Horsewill (a.horsewill@nottingham.ac.uk)
2. John Owers-Bradley (john.owers-bradley@nottingham.ac.uk)
Qualifications:
University of St Andrews, Master in Physics (Honours) Theoretical Physics
 Kelvin S K Goh
Kelvin S K GohRoom C20
School of Physics and Astronomy
University Park
NOTTINGHAM, NG7 2RD
Email: ppxkg@nottingham.ac.uk