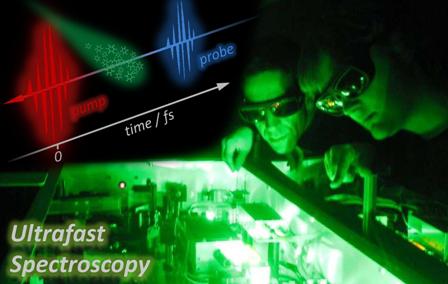
Research Overview
The interaction between femtosecond laser pulses (1 femtosecond = 10-15 seconds) and molecules has attracted considerable interest in recent years. The timescale on which atoms move and rearrange in molecules can take place as fast as femtoseconds, and so if one wishes to "capture" the temporal behaviour of chemical reactions femtosecond laser pulses must be utilised. The ability to follow chemical reactions using such ultrafast laser pulses led to the 1999 Nobel Prize in Chemistry to Ahmed Zewail [1] for his work on transition states of chemical reactions using femtosecond spectroscopy. Ultrafast lasers can be used as very fast cameras, allowing experimentalists to take snapshots of processes such as energy transfer in molecules. One example of this process is molecular bond dissociation. By observing how bonds are broken and formed, this can lead to very detailed insight into the mechanisms of chemical reactions.
Femtosecond pump-probe spectroscopy enables us to follow, in real time, vibrational motions coupled to electronic transitions. If the system is excited by a laser pulse shorter than the vibrational period, the vibrational coherence that is induced in both the ground and excited states provides detailed information about the nuclear dynamics of the excited state, and its evolution as a function of time.
In a pump-probe experiment, the output pulse-train from an ultrafast laser is used to generate two beams, the pump and probe. A femtosecond pulse (the pump) excites a sample of interest to some excited state and the changes it induces in the sample are probed by a second femtosecond pulse (the probe), which is suitably delayed with respect to the pump. Some property related to the probe (e.g. absorption or ionization) is then monitored as a function of the time delay to investigate the photochemical changes triggered by the pump in the sample.
Our research is conducted in both the gas and condensed phases. For more details on our specific research projects, please click on links above or take a look at the following references.
References:
1. Unravelling the photoprotective mechanisms of nature-inspired ultraviolet filters using ultrafast spectroscopy
T.T. Abiola, A.L. Whittock and V.G. Stavros
Molecules, 25 (2020) 3945
2. Applications of ultrafast spectroscopy to sunscreen development, from first principles to complex mixtures
E.L Holt and V.G. Stavros
Int. Rev. Phys. Chem., 38 (2019) 243-285
3. Unravelling photoprotection in microbial natural products
J.M. Woolley and V.G. Stavros
Science Progress, 102 (2019) 1-17
4. From fundamental science to product: a bottom-up approach to sunscreen development
N.D.N. Rodrigues and V.G. Stavros
Science Progress, 101 (2018) 8-31
5. Photoprotection: extending lessons learned from studying natural sunscreens to the design of artificial sunscreen constituents
L.A. Baker, B. Marchetti, T.N.V. Karsili, V.G. Stavros and M.N.R. Ashfold
Chem. Soc. Rev., 46 (2017) 3770-3791
6. A perspective on the ultrafast photochemistry of solution-phase sunscreen molecules
L.A. Baker, S.E. Greenough and V.G. Stavros
J. Phys. Chem. Lett., 7 (2016) 4655-4665
7. Photophysics of sunscreen molecules in the gas-phase: a stepwise approach towards understanding and developing next generation sunscreens
N.D.N. Rodrigues, M. Staniforth and V.G. Stavros
Proc. R. Soc. A., 472 (2016) 1-29
8. Observing and understanding the ultrafast photochemistry in small molecules: applications to sunscreens
L.A. Baker and V.G. Stavros
Science Progress, 99 (2016) 282-311
9. Gas-phase femtosecond particle spectroscopy: a bottom-up approach to nucleotide dynamics
V.G. Stavros and J. R. R. Verlet
Ann. Rev. Phys. Chem., 67 (2016) 211-232
10. The role of πσ* states in the photochemistry of heteroaromatic biomolecules and their subunits: insights from gas-phase femtosecond spectroscopy
G.M. Roberts and V.G. Stavros
Chem. Sci., 5 (2014) 1698-1722