Spectroelectrochemistry
Steady State and Ultrafast Spectroelectrochemistry Analysis of Red-Ox Active Compounds
The use of cyclic voltammetry and chronoamperometry in combination with absorption spectroscopy provides a powerful tool in the identification and characterisation of redox active molecules and the processes involved in the reaction mechanisms.
This was done by developing a custom built spectrophotometric cuvette (Figure 1) which can hold a three electrode system comprising:
-
a working electrode – the chosen potential is applied in a controlled way such as that the transfer of charge is facilitated to and from the analyte of interest present in solution;
-
a counter (auxiliary) electrode – used to pass enough current in order to balance the current measured at the working electrode;
-
a reference electrode – used as a reference in controlling and measuring the voltage passed at the working electrode as it maintains a constant potential and it does not pass any current.
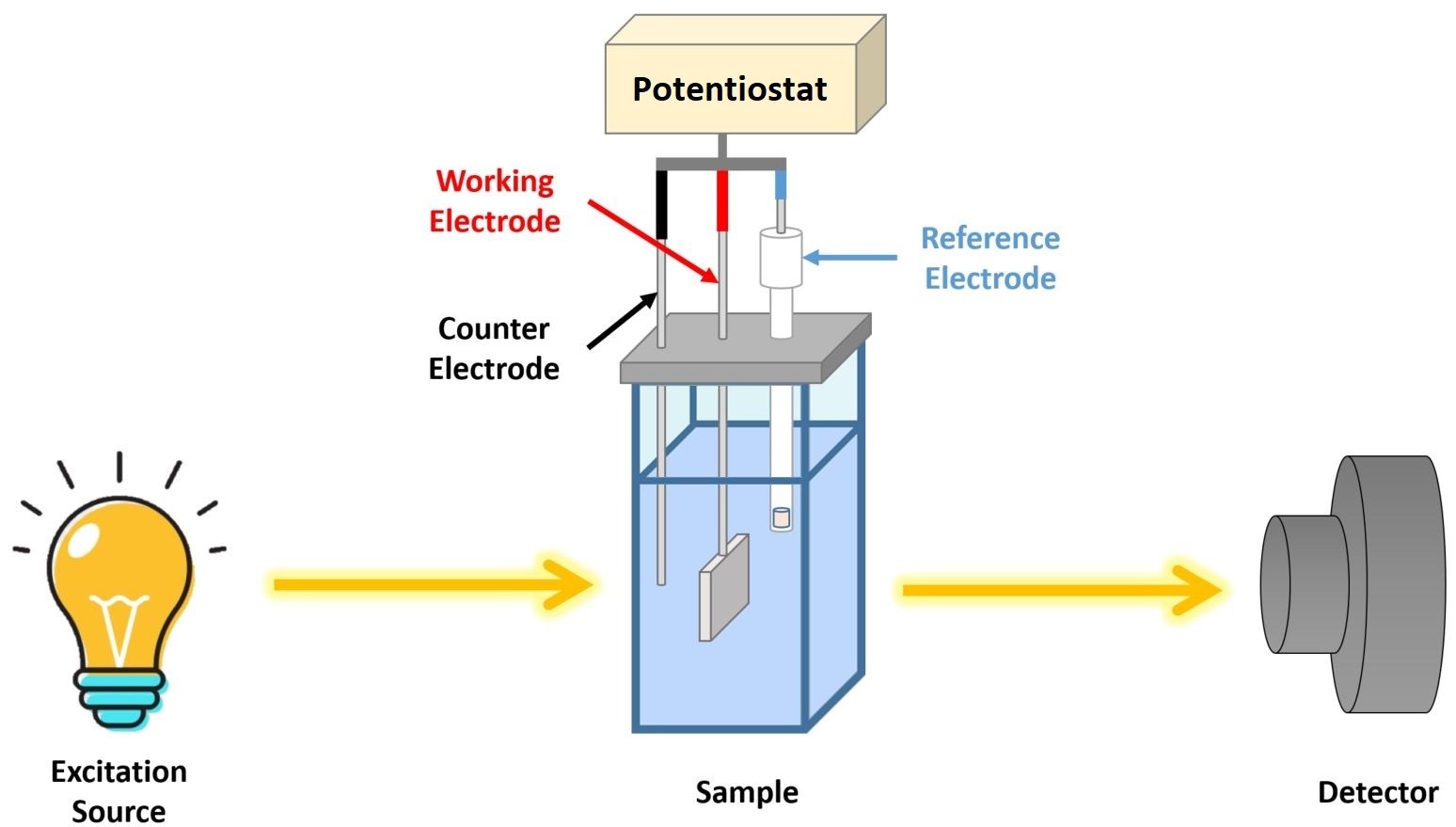
Figure 1: Schematic Diagram of the Spectroelectrochemical Instrumentation
The desired potential is chosen through cyclic voltammetry measurements in which the potential at the working electrode is linearly ramped against time, with a typical cyclic voltammogram being plotted as the current recorded (y axis/µA) against the applied potential (x axis/V) (Figure 2). Once the reduction potential (in our case) of the analyte of interest is determined, chronoamperometry is used to hold the chosen reduction potential that is applied at the working electrode, while the resulting current is measured against time. Thus, the generated reduced species can be analysed through steady state (Figure 2 inset) and ultrafast spectroscopic techniques as long as a potential is applied.
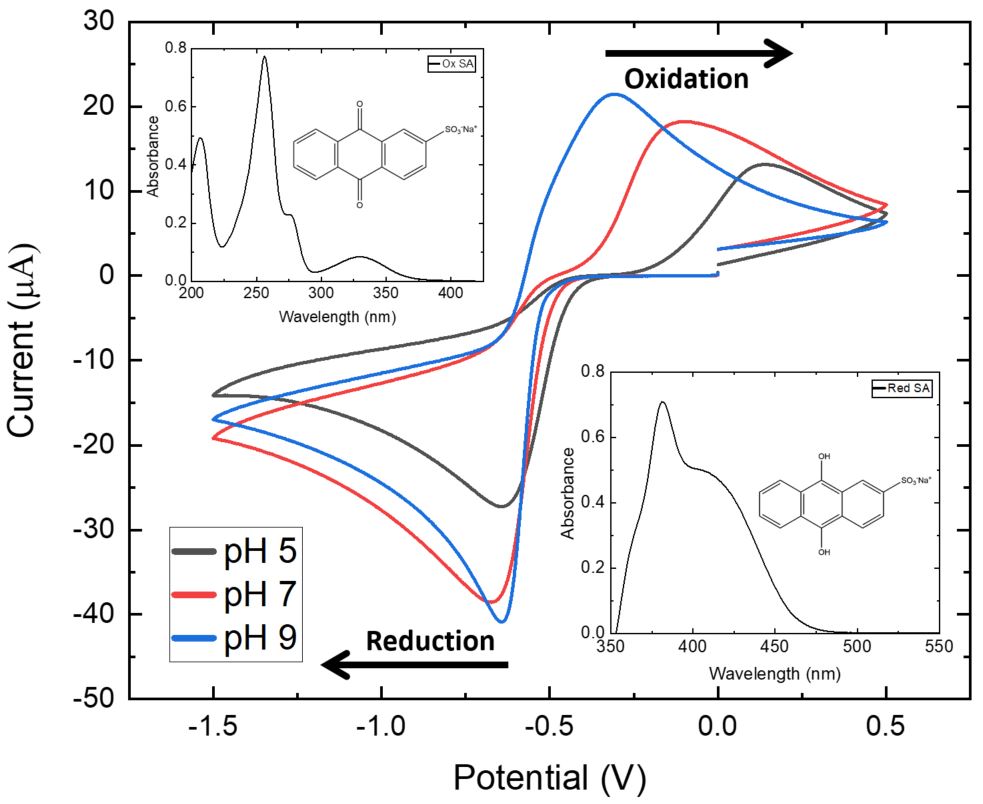
Figure 2: Cyclic Voltammogram (0.1 V/s scan rate, SCE reference electrode) recorded for sodium anthraquinone-2-sulfonate (SA) at different pH values highlighting the reduction (Red SA) peak and the oxidation peak (Ox SA) along with the corresponding UV/Vis spectra for each species (see inset spectra)
References
1. Elgrishi, N., Rountree, K.J., McCarthy, B.D., Rountree, E.S., Eisenhart, T.T, and Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2018, 95, 197−206
2. Cobb, S.J., Ayres, Z.J., and Macpherson, J.V. Boron Doped Diamond: A Designer Electrode Material for the Twenty First Century. Annu Rev Anal Chem (Palo Alto Calif). 2018, 11(1), 463-484
3. Gale, R.J. Spectroelectrochemistry: Theory and Practice, New York: Plenum Press, 1988
