Metals in Medicine: Research
Antiviral and Stem-Cell Mobilising Metallomacrocycles |
||
|
Our research concerns the design and study of the mechanism of action of anti-HIV and stem-cell mobilising metallomacrocycles. In particular, we are interested in the structure and reactivity of metal complexes of cyclams, bicyclams and derivatives thereof. Understanding the rich metal coordination chemistry of these metallomacrocycles is of prime importance as there is current medical interest in the bicyclam drug AMD3100, a potent anti-HIV agent and stem-cell mobiliser (see below). In our lab, we study the metal uptake of various cyclams and try to determine the structure in the solid state and, more importantly, the structure and dynamics in solution under physiologically-relevant conditions. We also study the binding of the metallocyclams to proteins to obtain more insight into the factors that govern receptor recognition, which is important for its biological activity. The principal techniques used to study these complexes are multidimensional, multinuclear NMR, (protein) X-ray crystallography, advanced mass spectrometry, molecular modelling studies and assays of viral infection.
|
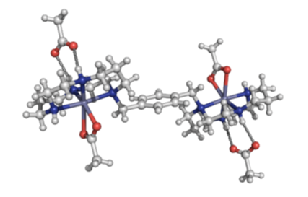
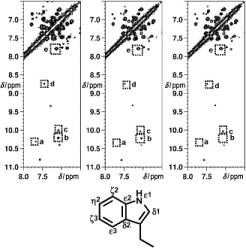
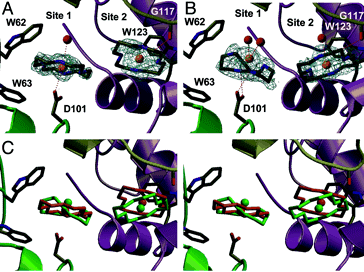
 |
|
|
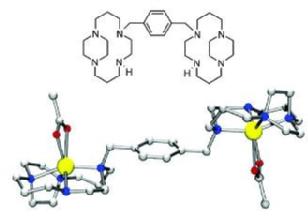
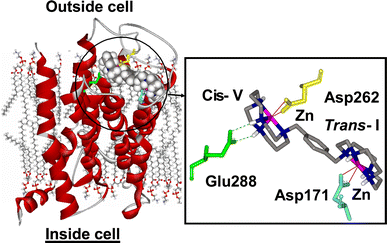
The Bicyclam Drug AMD3100 AMD3100 (Mozobil, the octa HCl salt of 1-1’-[1,4-phenylenebis(methylene)-bis(1,4,8,11-tetraazacyclotetradecane) has recently completed phase III clinical trials and attracts interest for its ability to mobilise hematopoietic progenitor cells from marrow to peripheral blood. The drug has also been in clinical trials as a highly potent and selective anti-HIV agent, but significant side-effects led to its withdrawal after phase II trials. In both applications the mechanism of action involves the specific binding of the bicyclam to the CXCR4 co-receptor. CXCR4 is a G-protein-coupled receptor which assists the entry of HIV into cells and anchors stem cells in the bone marrow. Receptor mutagenesis studies provide strong evidence for the involvement of several carboxylate side chains (Asp262, Glu288 and Asp171) in the bicyclam blocking of CXCR4 [1,2]. For some general reviews on AMD3100 see:
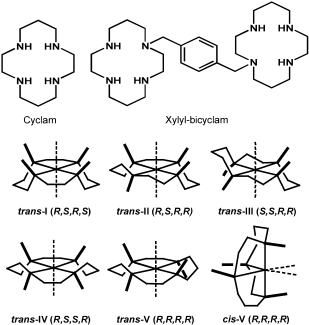
Metal-Binding Studies: Affinity, Structure and Configurational Dynamics Cyclams are strong metal-chelating agents and the anti-HIV activity of AMD3100 was found to be dependent on metal complexation in the following way: Zn2 > AMD3100 = Ni2 > Cu2 > Co2 >> > Pd2 Upon metal complexation the cyclam can adopt a number of different configurations which differ in the chirality of the bound N-donor atoms. The adopted configuration is metal-dependent and has a profound influence on the binding affinity towards the CXCR4 co-receptor, as it determines the possible interactions with amino acid side-chains, e.g. the previously mentioned carboxylates, and the proper orientation of the N-H hydrogens for additional hydrogen-bonding interactions. In solution, cyclam complexes can exist as complicated mixtures of isomers in dynamic equilibrium. A major part of our work on these metallomacrocyles therefore involves detailed 1D and 2D homo- and heteronuclear NMR studies to determine the solution structure and configurational dynamics of (bi)cyclams with different biologically-relevant metals. The observation that the Zn(II) complex of the bicyclam interacts 10 times more strongly with CXCR4 than AMD3100 has led to the suggestion that the Zn(II) complex is an important antagonist in vivo. We have studied the kinetics of zinc binding to cyclams under physiologically-relevant conditions and found that Zn(II)-uptake is relatively rapid, faster even than Cu(II) binding. These results showed that it is likely to be both thermodynamically- and kinetically-favourable for cyclam anti-HIV drugs to form Zn(II) complexes in blood plasma. [3] Zinc-(bi)cyclam studies Solution NMR studies showed that addition of acetate (to mimic the interaction with the carboxylate side chains of CXCR4) to a Zn(II)-bicyclam complex induced major structural changes resulting in a cis-V/trans-I arrangement as the major configuration. Docking studies of such a cis-V/trans-I Zn(II)-bicyclam complex onto an homology model of CXCR4 showed that specific binding interactions of the cis-V site with Asp262 (Zn coordination) and Glu288 (double H-bonding) and of the trans-I site with Asp171 (Zn bonding) could be formed. These combined interactions would allow for a strong induced-fit recognition and are consistent with the high anti-viral activity of the Zn2-bicyclam complex. Further experiments showed that the configuration in aqueous solution is highly anion-dependent and that addition of acetate indeed stabilizes the rather unusual cis-V configuration. The importance of the combination of direct coordinative binding and additional hydrogen bonding interactions is further illustrated by the crystal structure of the Zn2-bicyclam acetate complex in which both cyclam rings are found in the cis-V configuration [4,5]. Nickel-(bi)cyclam studies Since nickel enhances the binding strength of AMD3100 to CXCR4 even more than zinc does, we also studied the configurational equilibrium of Ni(II)-bicyclam complexes. The results suggest that cyclam complexes with nickel convert from either trans-III or cis-V configurations to a trans-I configuration, albeit much more slowly in the case of a cis-V starting configuration. Studies on the bicyclam system were more convoluted, but the results suggest that Ni2-bicyclam complexes can readily adopt a cis-V configuration in solution, which may account for the high antiviral activity of these nickel compounds. For this metal, the counterion appears to have little effect on the overall configuration of the nickel-cyclams in solution. [6] Protein Recognition of Macrocycles: Binding to Lysozyme We have also reported the first structures of protein adducts of metal cyclams. Lysozyme was chosen as a model protein as it readily crystallizes and the active site contains two carboxylates in close proximity. The interaction of Zn-bicyclam with lysozyme was demonstrated by ESI-MS and for Zn-cyclam by 2D [1H, 15N] NMR spectroscopy. The latter experiments showed shifts only for the trans-I and cis-V configurations upon lysozyme binding, which suggest that lysozyme is a useful model for the identification of specific interactions relevant to metallocyclam-CXCR4 binding. Solution studies using copper(II)-cyclam as a paramagnetic probe showed that the complexes interact with specific tryptophan residues of lysozyme. X-ray diffraction studies of Cu-cyclam and Cu-bicyclam protein adducts finally revealed two major binding sites, with both cis and trans configured cyclam rings. The crystal structures show that different combinations of metal coordination, hydrogen bonding and hydrophobic interactions determine the configurations of the cyclam rings. Similar observations were made both in solution and in X-ray diffraction studies of Ni-(bi)cyclam with lysozyme [6,7]. Configurationally Restricted Metallomacrocycles On the basis of these results, we anticipated that configurationally fixed AMD3100 analogues would have the advantage of presenting only one configuration in solution for binding to the protein. In a collaboration with the University of Hull, Archibald et al. constructed a configurationally-restricted bismacrocyclic compound and its zinc(II) complex. It was shown by high field NMR studies that only one configuration was adopted in solution, equivalent to a trans-II arrangement. Interestingly, the restricted Zn(II) complex was found to be more active against HIV infection in vitro than both AMD3100 and Zn2AMD3100.[8] |
|||
|
|
|||
|
|
|||
|
|
|||
|
|
|||
|
|
|||
|
|
|||
| Selection of our recent publications on antiviral and stem-cell mobilising metallomacrocycles: | ||
1. |
Liang XY, Campopiano DJ, Sadler PJ: Metals in membranes. Chemical Society Reviews 2007, 36:968-992. | |
2. |
Liang XY, Sadler PJ: Cyclam complexes and their applications in medicine. Chemical Society Reviews 2004, 33:246-266. | |
3. |
Paisey SJ, Sadler PJ: Anti-viral cyclam macrocycles: rapid zinc uptake at physiological pH. Chemical Communications 2004:306-307. | |
4. |
Liang XY, Parkinson JA, Weishaupl M, Gould RO, Paisey SJ, Park HS, Hunter TM, Blindauer CA, Parsons S, Sadler PJ: Structure and dynamics of metallomacrocycles: Recognition of zinc xylyl-bicyclam by an HIV coreceptor. Journal of the American Chemical Society 2002, 124:9105-9112. | |
5. |
Liang XY, Weishaupl M, Parkinson JA, Parsons S, McGregor PA, Sadler PJ: Selective recognition of configurational substates of zinc cyclam by carboxylates: Implications for the design and mechanism of action of anti-HIV agents. Chemistry-a European Journal 2003, 9:4709-4717. | |
6. |
Hunter TM, McNae IW, Simpson DP, Smith AM, Moggach S, White F, Walkinshaw MD, Parsons S, Sadler PJ: Configurations of nickel-cyclam antiviral complexes and protein recognition. Chemistry-a European Journal 2007, 13:40-50. | |
7. |
Hunter TM, McNae IW, Liang XY, Bella J, Parsons S, Walkinshaw MD, Sadler PJ: Protein recognition of macrocycles: Binding of anti-HIV metallocyclams to lysozyme. Proceedings of the National Academy of Sciences of the United States of America 2005, 102:2288-2292. | |
8. |
Valks GC, McRobbie G, Lewis EA, Hubin TJ, Hunter TM, Sadler PJ, Pannecouque C, De Clercq E, Archibald SJ: Configurationally restricted bismacrocyclic CXCR4 receptor antagonists. Journal of Medicinal Chemistry 2006, 49:6162-6165. | |
9. |
Hunter TM, Paisey SJ, Park HS, Cleghorn L, Parkin A, Parsons S, Sadler PJ: Configurations of metallocyclams and relevance to anti-HIV activity. Journal of Inorganic Biochemistry 2004, 98:713-719. | |
10. |
Liang XY, Parkinson JA, Parsons S, Weishaupl M, Sadler PJ: Cadmium cyclam complexes: Interconversion of cis and trans configurations and fixation of CO2. Inorganic Chemistry 2002, 41:4539-4547. |
|