Modelling and Simulation
Intrinsically disordered regions (IDRs)
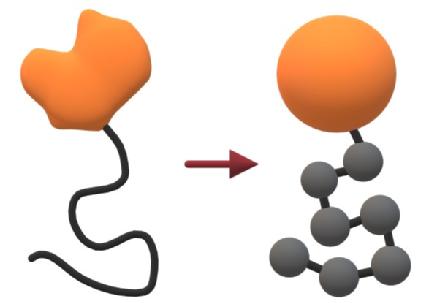
A consistent feature of membraneless organelles is the presence of proteins containing IDRs, which are sections of the polypeptide chain that remains flexible instead of folding as per the classic protein paradigm. In essence, proteins with IDRs can be viewed as classic, well-structured proteins with one or more flexible polymers attached.
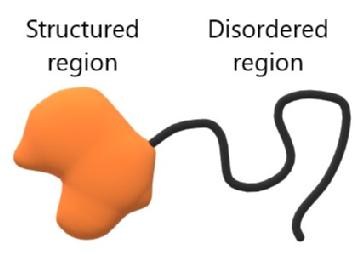
While IDRs do not have the structure to facilitate specific interactions, e.g. via the lock-and-key model, they contain charged or polar amino acids which lead to weak attractive forces that are believed to be central to their role in clustering IDR-containing proteins in membraneless organelles.
The role of entropy
While the weak IDR-IDR interactions no doubt play a part, they overlook a more fundamental possible driver of biological phase separation: entropy. Though usually cited as a cause of disorder, there are systems in which entropy alone is known to order systems and drive phase separation. One such system is a mixture of polymers and colloids, which is close enough to our biological system to motivate modelling the system without the IDR-IDR interactions to determine whether they or entropy is the dominant driver.
Thus we model the proteins using established polymer and colloid physics in the absence of attractive forces, with the key additional feature that the two are bonded together.