Seminar: Octahedral tilting in Prussian blue analogues, Dr Hanna Boström
 ‘Octahedral tilting in Prussian blue analogues’ will be presented by Dr. Hanna Boström via MS Teams at 12.00, on Thursday 28 October, 2021. (Note the seminar is earlier than usual).
‘Octahedral tilting in Prussian blue analogues’ will be presented by Dr. Hanna Boström via MS Teams at 12.00, on Thursday 28 October, 2021. (Note the seminar is earlier than usual).
 Hanna Boström originates from Uppsala, Sweden but relocated to Scotland in 2011 to study Chemistry at the University of St Andrews.
Hanna Boström originates from Uppsala, Sweden but relocated to Scotland in 2011 to study Chemistry at the University of St Andrews.
In 2015, she started her DPhil studies in Inorganic Chemistry at the University of Oxford under the supervision of Prof. Andrew Goodwin, supported by an Oxford Graduate Scholarship.
Her first postdoctoral position was held at Uppsala University, and since February 2021, she works as a Humboldt postdoctoral researcher at the Max Planck Institute for Solid State Research in Germany. Her research focuses on the crystallography of coordination polymers and metal−organic frameworks.
________________________________________________________________________________
Octahedral tilting in Prussian blue analogues
Hanna L. B. Boström
1Max Planck Institute for Solid State Research, Germany h.bostroem@fkf.mpg.de
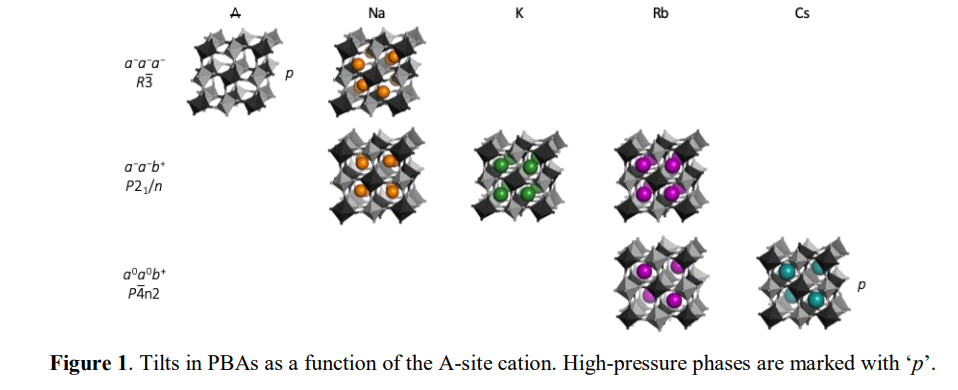
Prussian blue analogues (PBAs) are metal–cyanide frameworks with the formula AxM[Mʹ(CN)6]1−y□y⋅nH2O, where A is an alkali metal, M and Mʹ are transition metals and □ denotes a vacancy. Due to the large scope for compositional and stoichiometric variations, PBAs show promise for a wide range of applications, from catalysis to energy storage.1,2 The parent PBA structure adopts the cubic space group Fm3̅m, analogous to a double perovskite. Like perovskites, octahedral tilting has a strong impact on the symmetry and the functional response of PBAs. For example, tilts nearly triple the magnetic ordering temperature in the monoclinic Na2MnMn(CN)6 compared to the cubic Cs2MnMn(CN)6, 3 but also lead to undesirable phase transitions upon Na intercalation in cathode materials. 4 Despite its importance, the tilting in PBAs is poorly understood, which is evidenced by considerable confusion in the literature. A systematic understanding of the driving forces for octahedral tilting in PBAs would be valuable and facilitate tilt engineering approaches. Here, the link between structural features and octahedral tilting in PBAs is rationalised using literature surveys, group theory and high-pressure diffraction. A high concentration of intermediately sized A-site cations is a prerequisite for tilting and PBAs with x < 1 or A = Cs are invariably cubic, even upon cooling. However, external pressure can induce interesting tilted phases nearly irrespective of stoichiometry, such as the polar and potentially ferroelectric RbMnCo(CN)6. 5 In addition, the A-site cation radius dictates the particular tilt pattern [Fig. 1], in line with the behaviour of perovskites. Water also plays a particularly intriguing role: (de)hydration can affect the type of tilt that appears in response to either external or chemical pressure. More generally, the results help to develop a unified picture of the structural behaviour of PBAs and also improve the understanding of octahedral tilting distortions in general.
1. C. Marquez, M. Rivera-Torrente, P. P. Paalanen, B. M. Weckhuysen, F. G. Cirujano, D. De Vos and T. De Baerdemaeker, J. Catal., 2017, 354, 92–99.
2. W. J. Li, C. Han, G. Cheng, S. L. Chou, H. K. Liu and S. X. Dou, Small, 2019, 15, 1900470.
3. C. M. Kareis, S. H. Lapidus, J.-H. Her, P. W. Stephens and J. S. Miller, J. Am. Chem. Soc., 2012, 134, 2246–2254.
4. D. Asakura, M. Okubo, Y. Mizuno, T. Kudo, H. Zhou, K. Ikedo, T. Mizokawa, A. Okazawa, and N. Kojima, J. Phys. Chem. C, 2012, 116, 8364–8369.
5. H. L. B. Boström, I. E. Collings, D. Daisenberger, C. J. Ridley, N. P. Funnell, and A. B. Cairns, J. Am. Chem. Soc., 2021, 143, 3544–3554.
