Transition Metal Catalyst Design and Application in the Synthesis of Cyclic Polymers

13:45 - 14:45, Fri 16 Jun, 2023 OC1.05
Adam Veige received a Hons. BSc. degree in Chemistry in 1997 from the University of Western Ontario, Canada. Dr. Veige obtained his PhD. in 2003 at Cornell University while working with Dr. Peter T. Wolczanski. His PhD. research at Cornell focused on kinetic and mechanistic investigations of O-atom transfer and a rare metal-olefin to metal-alkylidene isomerization. His postdoctoral work under the guidance of Dr. Daniel G. Nocera, at the Massachusetts Institute of Technology, focused on elucidating the mechanism of photocatalytic H2 evolution from rhodium and iridium mixed-valent complexes. In 2004 he joined the Department of Chemistry at the University of Florida as Assistant Professor in Inorganic Chemistry. Dr. Veige’s manages a multidisciplinary chemistry group covering research in the areas of transition metal catalysis related to polymer synthesis, alkene and alkyne metathesis, metallopolymer synthesis, cancer targeting aptamer-organometallics, small molecule activation, and polymer recycling. Another area of current interest is the synthesis of in-chain metallopolymers using iClick technology invented by the Veige group. In 2010, Dr. Veige was named Director for the Center for Catalysis, and in 2015 he was promoted to Professor. In 2017 Dr. Veige was named a University of Florida Research Foundation Professor. Dr. Veige was awarded a Camille and Henry Dreyfus New Faculty Award, an Alfred P. Sloan Fellowship, an NSF Career award, the Dr. Paul Tarrant Fellowship, the University of Florida Undergraduate Student Mentor of the Year Award (2012), and the UF College of Liberal Arts Graduate Student Mentor of the ear Award (2019)
Abstract
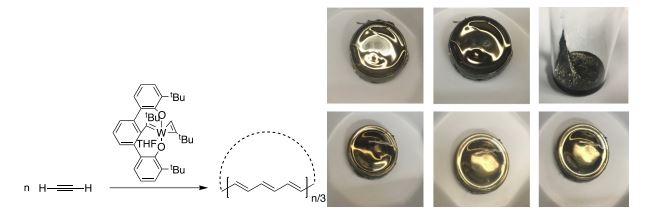
Employing a trianionic pincer ligand, we have discovered a highly active catalyst for the synthesis of cyclic polymers from alkynes. Though initiated with a trianionic pincer ligand supported tungsten catalyst, the active catalyst features a tetraanionic pincer ligand. Details of the catalyst design and a discussion of the polymerization mechanism and active catalyst elucidation will be provided. Access to these cyclic polymers enables the synthesis of commercially relevant polyolefins. Annually produced at a rate of 170 million tons per year, polyolefin manufacturing has changed human quality of life and planet Earth forever. Isotactic polypropylene comprises 25% of all polyolefins manufactured and is applied in countless products globally. Polypropylene and all industrially produced polyolefins are linear molecules containing chain-ends. Exploiting ring expansion polymerization of alkynes, atactic cyclic polypropylene can now be synthesized. Characterization and confirmation of a cyclic topology comes from size exclusion chromatography, dynamic light scattering, viscometry, and rheology. Importantly, the cyclic topology of polypropylene leads to dramatic differences in the physical properties of the polymer including a > 20 °C increase in its glass transition temperature (Tg) compared to the linear version. Additional polyolefins such as the bulk scale synthesis of cyclic poly-1-pentene, and the synthesis of a highly transparent cyclic version of the commodity polyolefin TPXTM, poly(4-methylpentene), will be discussed. Another challenge is to prepare stereoregular cyclic polymers. This seminar will discuss catalyst designs for the ring opening polymerization of norbornene to give cis and syndiotactic enriched cyclic polynorbornene. Featured in the catalyst designs are the concepts of an “Inorganic Enamine” and “Ynene Metathesis” and their relationship to accentuating the nucleophilicity of metal-carbon multiple bonds. Finally, in most recent work we report the synthesis of cyclic polyacetylene.