Metalated Ylides: From Space Molecules to Rocket-Fast Catalysts, Prof Viktoria Gessner

Metalated Ylides:
From Space Molecules to Rocket-Fast Catalysts
Prof Viktoria Gessner,
Ruhr-University Bochum
13:00 - 14:00
Wed 15 May, 2024
PLT
Prof Viktoria Gessner joins us to deliver this departmental seminar. All staff and students welcome. Refreshments will be served outside PLT at 12:45
Abstract
Since the discovery of ylides more than 100 years ago, this class of compounds has developed to versatile reagents in organic synthesis.[1] While early days were particularly coined by the advent of Wittig and Wittig-type olefination reactions,[2] they are nowadays applied in many stoichiometric and catalytic transformations.[3] Also their α-metalated congener - the so-called yldiides - have early attracted research interest due to their strong nucleophilicity and potential use as versatile building blocks. However, selective applications long remained challenging because of their high reactivity, often observed unselective and difficult preparation in larger scale.
In the past years, our group has reported on the synthesis and reactivity of α-metalated ylides (1). Owing to their highly nucleophilic character these compounds turned out to be excellent precursors to access electron-rich phosphines with beneficial properties for homogeneous catalysis[4] and to stabilize electron-deficient main group species.[5] Most recently, we uncovered that metalated ylides can also react as coordination complexes of carbon to readily exchange the phosphine substituent by carbon monoxide.[6] This remarkable reactivity provided access to reactive species such as ketenyl anions 2 which have long been regarded as fleeting intermediates, but proven sufficiently stable to serve as starting materials to a variety of carbonyl compounds. Here, we will summarize this unique reactivity of metalated ylides.
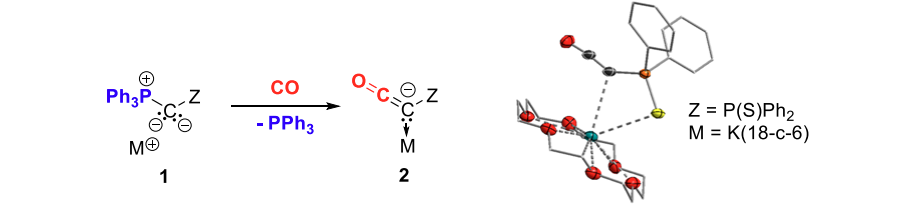
Figure 1. Phosphine by CO exchange in metallated ylides
References
[1] A. Michaelis, H. V. Gimborn, Ber. 1894, 27, 272.
[2] G. Wittig, G. Geissler, Liebigs Ann. 1953, 580, 44.
[3] O. I. Kolodiazhnyi in Phosphorus Ylides: Chemistry and Application in Organic Synthesis, Wiley-VCH, Weinheim, 1999.
[4] a) T. Scherpf, R. Wirth, S. Molitor, K.-S. Feichtner, V. H. Gessner, Angew. Chem. Int. Ed. 2015, 54, 8542. b) S. Lapointe, A. Sarbajna, V.H. Gessner, Acc. Chem. Res. 2022, 55, 770.
[5] A. Sarbajna, V.S.V.S.N. Swamy, V.H. Gessner, Chem. Sci. 2021, 12, 2016
[6] M. Jörges+, F. Krischer+, V. H. Gessner, Science, 2022, 378, 1331
