Costantini and Wills Groups on Cover of ChemComm
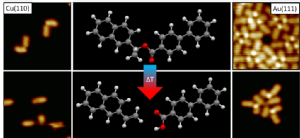 A novel chiral ester was synthesised by Zhijia Fang in the Wills group and was deposited on metallic substrates and measured through scanning tunnelling microscopy (STM) by Ben Moreton in the Costantini group. A direct comparison with possible products of dissociation allowed for the delineation of the cleavage mechanism as reported in ChemComm.
A novel chiral ester was synthesised by Zhijia Fang in the Wills group and was deposited on metallic substrates and measured through scanning tunnelling microscopy (STM) by Ben Moreton in the Costantini group. A direct comparison with possible products of dissociation allowed for the delineation of the cleavage mechanism as reported in ChemComm.
Ester cleavage is important for many applications, such as the hydrolysis of fatty acids to soap, the products of which can vary, typically, depending on the reaction conditions used. Here we utilise STM to investigate catalysis by model system metallic substrates, namely Cu(110) and Au(111).
(S)-1-(2’-naphthyl)ethyl-(4-phenylbenzoate) was synthesised by Zhijia Fang in the Wills group was imaged showing its absolute chirality on low temperature Cu(110) and room temperature Au(111). On the Au the molecules arrange hierarchically, initially trimers are formed via H-bonding which then aggregate further via van der Waals interactions.
