Michael Horbury (PDRA)

Contact Details
Email: M.Horbury@warwick.ac.uk
Telephone: +44(0)2476151013
Office: E0.11a
Lab: E0.19
Project Details
Biological systems have always had to contend with the ultraviolet radiation produced by the sun, which has led to a myriad of mechanisms to deal with any deleterious effects. While many intrinsic defence mechanisms exist within biomolecules, they are not perfect, therefore many biological systems employ molecules that preferentially absorb ultraviolet radiation, i.e. melanin pigments in human skin. However, yet again, this photoprotective mechanism has flaws, a delayed response, and in humans this can lead to sunburn or in severe cases, skin cancer. This had led to the use of artificial sunscreens to bolster the photoprotection of the human skin and avoid these dangerous side effects of sun exposure.
In recent years several synthetic molecules used within commercial sunscreen formula have come under scrutiny, with several being found to be phototoxic. This has led to the idea of exploring the photodynamics in other natural photoprotective biomolecules, like sinapate esters (a sub class of hydroxycinnamates), to potentially provide a new avenue towards making superior synthetic sunscreens.
To observe the photochemistry of these sunscreens, femtosecond resolution spectroscopy is required, therefore I use condensed-phase femtosecond transient electronic (UV/visible) absorption spectroscopy to do this. This technique has given unique insight into several synthetic sunscreen molecules, as well as several natural photoprotective molecules. My current work has focussed mainly on sinapate esters, the photoprotective molecules found in plant leaves.
Recently, building off the work on sinapate esters, I have been exploring the photochemistry of a series of synthetic molecules with the potential to be new sunscreening agents.
While there is a plethora of photoprotective mechanisms, both natural and synthetic, they can still fail, leading to, in the worst-case scenario, skin cancer. Therefore, I am interested in molecules that can be employed as photoactivated anti-cancer treatments; particularly ones that can be activated through two-photon excitation.
Publications
Selected Publication:
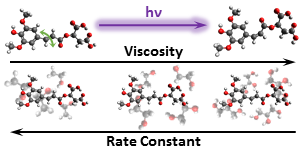
Elucidating nuclear motions in a plant sunscreen during photoisomerization through solvent viscosity effects
Michael D. Horbury, Wen-Dong Quan, Amandine L. Flourat, Florent Allais, and Vasilios G. Stavros, Phys. Chem. Chem. Phys., 2017, 19, 21127
Publication List:
Ultrafast photo-induced ligand solvolysis of cis-[Ru(bipyridine)2(nicotinamide)2]2+: experimental and theoretical insight into its photoactivation mechanism
Simon E. Greenough, Gareth M. Roberts, Nichola A. Smith, Michael D. Horbury, Russell G. McKinlay, Justyna M. Żurek, Martin J. Paterson, Peter J. Sadler, and Vasilios G. Stavros, Phys. Chem. Chem. Phys., 2014, 16, 19141
Solvent induced conformer specific photochemistry of guaiacol
Simon E. Greenough, Michael D. Horbury, James O. F. Thompson, Gareth M. Roberts, Tolga N. V. Karsili, Barbara Marchetti, Dave Townsend, and Vasilios G. Stavros, Phys. Chem. Chem. Phys., 2014, 16, 16187
Bridging the gap between the gas and solution phase: solvent specific photochemistry in 4-tert-butylcatechol
Michael D. Horbury, Lewis A. Baker, Wen-Dong Quan, Jamie D. Young, Michael Staniforth, Simon E. Greenough, and Vasilios G. Stavros, J. Phys. Chem. A., 2015, 119, 11989
Probing the ultrafast energy dissipation mechanism of the sunscreen oxybenzone after UVA irradiation
Michael D. Horbury, Lewis A. Baker, Simon E Greenough, Philip M Coulter, Tolga N. V. Karsili, Gareth M. Roberts, Andrew J. Orr-Ewing, Michael N. R. Ashfold, and Vasilios G. Stavros, J. Phys. Chem. Lett., 2015, 6, 1363
Broadband ultrafast photoprotection by oxybenzone across the UVB and UVC spectral regions
Lewis A. Baker, Michael D. Horbury, Simon E. Greenough, Michael N. R. Ashfold, and Vasilios G. Stavros, Photochem. Photobiol. Sci., 2015, 14, 1814
Ultrafast photoprotecting sunscreens in natural plants
Lewis A. Baker, Michael D. Horbury, Simon E. Greenough, Florent Allais, Patrick S. Walsh, Scott Habershon, and Vasilios G. Stavros, J. Phys. Chem. Lett., 2016, 7, 56
Ultrafast photoprotective properties of the sunscreening agent octocrylene
Lewis A. Baker, Michael D. Horbury, and Vasilios G. Stavros, Optics Express, 2016, 24, 10700
Photodynamics of potent antioxidants: ferulic and caffeic acids
Michael D. Horbury, Lewis A. Baker, Wen-Dong Quan, Simon E. Greenough, and Vasilios G. Stavros, Phys. Chem. Chem. Phys., 2016, 18, 17691
Excited-state dynamics of a two-photon-activatable ruthenium prodrug
Simon E. Greenough, Michael D. Horbury, Nichola A. Smith, Peter J. Sadler, Martin J. Paterson, and Vasilios G. Stavros, ChemPhysChem, 2016, 17, 221
Combatting AMR: photoactivatable ruthenium (ii)-isoniazid complex exhibits rapid selective antimycobacterial activity
Nichola A. Smith, Pingyu Zhang, Simon E. Greenough, Michael D. Horbury, Guy J. Clarkson, Daniel McFeely, Abraha Habtemariam, Luca Salassa, Vasilios G. Stavros, Christopher G. Dowson, and Peter J. Sadler, Chem. Sci., 2017, 8, 395
Photoisomerization of ethyl ferulate: A solution phase transient absorption study
Michael D. Horbury, Lewis A. Baker, Natércia D. N. Rodrigues, Wen-Dong Quan, and Vasilios G. Stavros, Chem. Phys. Lett., 2017, 673, 62
Observation of excited state absorption in the V-Cr Prussian blue analogue
Luke Hedley, Michael D. Horbury, Florian Liedy, and J. Olof Johansson, Chem. Phys. Lett., 2017, 687, 125
Controlled fabrication of osmium nanocrystals by electron, laser and microwave irradiation and characterisation by microfocus X-ray absorption spectroscopy
Anaïs Pitto-Barry, Kalotina Geraki, Michael D. Horbury, Vasilios G. Stavros, J. Frederick W. Mosselmans, Richard I. Walton, Peter J. Sadler and Nicolas P. E. Barry, Chem Comm, 2017, 53, 12898
Photophysics of the sunscreen ingredient menthyl anthranilate and its precursor methyl anthranilate: A bottom-up approach to photoprotection
Natércia D. N. Rodrigues, Neil C. Cole-Filipiak, Michael D. Horbury, Michael Staniforth, Tolga N. V. Karsili, Yoann Peperstraete, and Vasilios G. Stavros, J. Photochem. Photobiol. A, 2017, 353, 376
Investigating isomer specific photoprotection in a model plant sunscreen
Michael D. Horbury, Amandine L. Flourat, Simon E. Greenough, Florent Allais, and Vasilios Stavros, Chem. Comm., 2018, 54, 936
Spectroscopic studies on photoinduced reactions of the anticancer prodrug, trans,trans,trans-[Pt(N3)2(OH)2(py)2]
Robbin R. Vernooij, Tanmaya Joshi, Michael D. Horbury, Bim Graham, Ekaterina I. Izgorodina, Vasilios G. Stavros, Peter J.Sadler, Leone Spiccia, and Bayden R. Wood, Chem. Eur. J., 2018, 24, 1
Unravelling the photoprotection properties of mycosporine amino acid motifs
Jack M. Woolley, Michael Staniforth, Michael D. Horbury, Gareth W. Richings, Martin Wills and Vasilios G. Stavros, J. Phys. Chem. Letts., 2018, 9, 3043
Determination of Secondary Species in Solution through Pump-Selective Transient Absorption Spectroscopy and Explicit-Solvent TDDFT
Matthew A. P. Turner, Michael D. Horbury, Vasilios G. Stavros and Nicholas D. M. Hine, J. Phys. Chem. A, 2019, 123, 873
The Role of Symmetric Functionalisation on Photoisomerisation of a UV Commerical Chemical Filter
Jack M. Woolley, Jack S. Peters, Matthew A. P. Turner, Guy J. Clarkson, Michael D. Horbury and Vasilios G. Stavros, Phys. Chem. Chem. Phys., 2019, DOI: 10.1039/C8CP06536E
