Recent Developments in the Patho-Physiological Molecular Clocks Lab
CCC - Clock Club Community
Based on an initiative by former Warwick academic Prof Andrew Millar (Edinburgh) at the UK Clock Club in Oxford we are trying to further develop the circadian community in the UK. Have your say here:
New students in the lab!
We welcome back Rachael Ralph (MRC DTP mini project student) who will work on a collaborative project with Sam Dean on Trypanosoma brucei.
Furthermore, we welcome Luke Reynoldson (MIBTP mini project) has joined us to ask questions about the fitness of cells with clock gene disruptions.
We won a 6-months loan of an nCounter Sprint
Kudos to Robert Dallmann, from the @warwickuni for winning the nCounter loan Grant! 🎖️
— NanoString (@nanostringtech) April 9, 2022
He will use the nCounter SPRINT in his research to find new biomarkers to stratify breast cancer patients. We look forward to lots of gene expression studies coming out of Warwick! pic.twitter.com/KWwjkZooM7
Your Life and Cancer workshop on the impact of clocks on cancer
In discussion together with Dr Sam Watts, Robert discussed the impact of the potentially vicious cycle of clock disruption and chronic disease in a session on: "Circadian Rhythms – how clock disruption can lead and influence disease progression" at the Your Life and Cancer 2022 conference.(https://www.yourlifeandcancer.com).
Cell-type specific circadian bioluminescence rhythms in Dbp reporter mice
Collaborative project foremost with David Weaver, UMassMed, who had the will to stick out making this mouse based on one of these "wouldn't it be nice" discussions. With important contributions from Mary Harrington's, Alec Davidson's groups and Tanya Leise.
Finally out, the tissue specific DBP-luc reporter model in JBR with heroic contribution of Lauren Garbutt who characterised the fluorescence reporter part of this mouse. We will add to the characterisation of the mice and the reporter construct in the not too far future and have found an these reporter model to be ideal to further address tissue and cell type specific circadian rhythms.
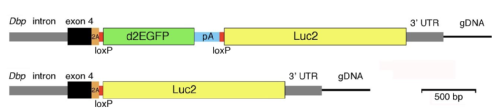
Circadian rhythms are endogenously generated physiological and molecular rhythms with a cycle length of about 24 h. Bioluminescent reporters have been exceptionally useful for studying circadian rhythms in numerous species. Here, we report development of a reporter mouse generated by modification of a widely expressed and highly rhythmic gene encoding D-site albumin promoter binding protein (Dbp). In this line of mice, firefly luciferase is expressed from the Dbp locus in a Cre recombinase-dependent manner, allowing assessment of bioluminescence rhythms in specific cellular populations. A mouse line in which luciferase expression was Cre-independent was also generated. The Dbp reporter alleles do not alter Dbp gene expression rhythms in liver or circadian locomotor activity rhythms. In vivo and ex vivo studies show the utility of the reporter alleles for monitoring rhythmicity. Our studies reveal cell-type-specific characteristics of rhythms among neuronal populations within the suprachiasmatic nuclei ex vivo. In vivo studies show Dbp-driven bioluminescence rhythms in the liver of Albumin-Cre;DbpKI/+ “liver reporter” mice. After a shift of the lighting schedule, locomotor activity achieved the proper phase relationship with the new lighting cycle more rapidly than hepatic bioluminescence did. As previously shown, restricting food access to the daytime altered the phase of hepatic rhythmicity. Our model allowed assessment of the rate of recovery from misalignment once animals were provided with food ad libitum. These studies confirm the previously demonstrated circadian misalignment following environmental perturbations and reveal the utility of this model for minimally invasive, longitudinal monitoring of rhythmicity from specific mouse tissues.
