Applications to Pharamaceuticals and Supramolecular Chemistry
2D MAS NMR experiments reveal details of how organic molecules pack in the solid-state. High-resolution 1H experiments (Brown 2012) are particularly powerful given the importance of intermolecular hydrogen bonding and CH-π interactions in determining the adopted self assembly.
A recent Knowledge Transfer Partnership with AstraZeneca and the University of Warwick (with Pat Szell, Józef Lewandowski, and Steven Brown) to mitigate some of the problems of crystallographic disorder in active pharmaceutical ingredients has been rated as outstanding. Read the case study here.
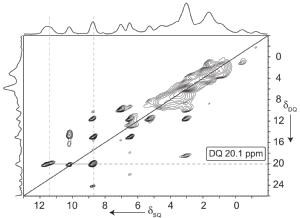
In collaboration with Astra Zeneca, we have shown how 1H-1H DQ (double-quantum) CRAMPS 2D spectra represent fingerprints of the anhydrous and hydrous form of an active pharmaceutical ingredient (API), enabling the identification of the anhydrous form in a tablet formulation containing the API and excipients (Griffin 2007).
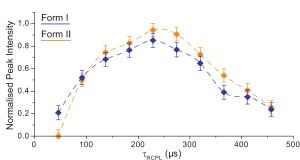
We have further shown how 1H DQ build-up curves that plot the change in intensity of specific DQ peaks as a function of the DQ recoupling time reveal subtle differences for two polymorphs of the API viozan that is related to differences in inter-planar packing arrangements. (Bradley 2012)
In collaboration with GlaxoSmithKline, we have demonstrated the applicability of 2D 14N-1H experiments for probing intermolecular hydrogen bonding in the API cimetidine (Tatton 2012). This work has further been extended to co crystals and amorphous dispersions. (Maruyoshi 2012, Tatton 2013)
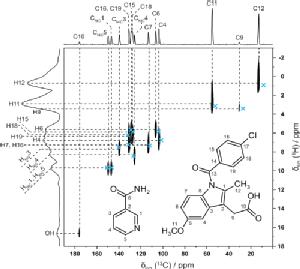
In collaboration with Professor Kenneth Harris, Cardiff University we have shown how NMR crystallography (CCPNC) can be used in synergy with structure solution by powder X-ray diffraction diffraction for an indomethacin-nicotinamide co-crystal – note the excellent 2D agreement with calculated (GIPAW, blue crosses) 13C and 1H chemical shifts for directly bonded CHs. (Dudenko 2013).
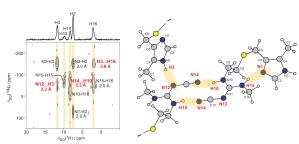
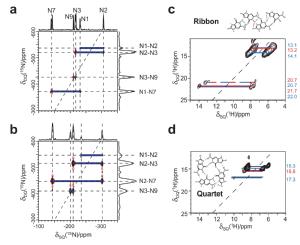
We have shown how different intermolecular hydrogen-bonding arrangements associated with distinct modes of self-assembly exhibited by guanosine derivatives synthesized in the group of Prof. Spada (Bologna, Italy) can be distinguished using 15N-15N refocused INADEQUATE experiments (for 15N-labelled derivatives (Pham 2005)) or 1H-1H DQ CRAMPS experiments (for derivatives at natural isotopic abundance (Webber 2011)).