Why should we make vehicles electric?
Why should we make vehicles electric?
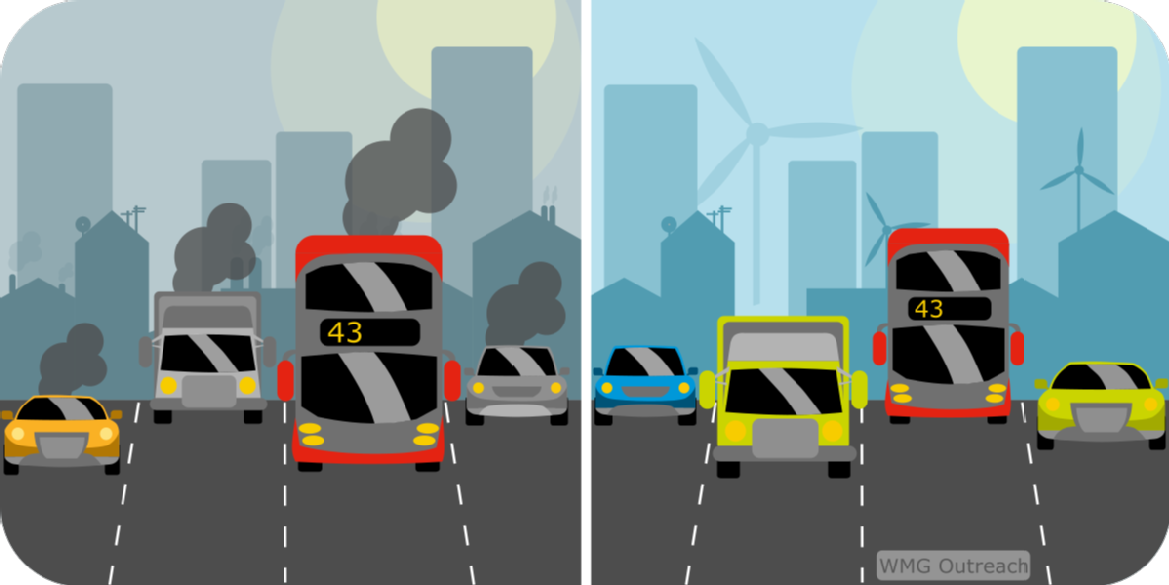
What will the cities of the future look like?
 Introduction
Introduction
I bet you’ve heard someone mention an electric vehicle at some point. This series of posts is going to go from absolutely zero knowledge about cars up to real expert knowledge – so fasten your seatbelts and let’s start our engines.
If you had to build a car now, what would you use to power it? Maybe you could add a sail like a boat and wait for wind? Or perhaps some kind of wind-up power like clockwork? Our engineers have actually tried powering cars using some unusual ideas over at Engineer Inside.
 Focus
Focus
So there are different ways to power a vehicle – what’s the problem with the way that cars have worked for years (petrol or diesel) and why are we spending so much time, effort and money developing electric vehicles?
To start with, we need to understand the problems with using petrol or diesel…
How do "normal" cars work?
A “normal” car runs on petrol or diesel and it take a little bit of fuel, turns it into a gas in a chamber in the engine and then sets fire to it to make it explode! The force of the explosion is used to move the car along a tiny bit, so thousands of explosions are needed every minute to actually get the car up to speed. Energy is stored in the fuel, it is released by the chemical reactions that take place as the fuel burns, and it is used to move the car along.
 |
Unfortunately the faster you try and get something done the less efficient it is, and an explosion is just about the fastest chemical reaction it is possible to make. Imagine you’re trying to stack blocks up – you can do it very efficiently when you do them one at a time and get exactly the result you want but if you rush and just chuck five of them in the air you might get a couple on top of each other but not quite in the order you want them! |
In terms of a chemical reaction, we’re trying to break apart a long chain of carbon atoms into chunks with just one carbon atom and two oxygen atoms – carbon dioxide. All the hydrogen atoms in the chain react with more oxygen and we create water. While carbon dioxide is bad for climate change, this is actually the dream outcome of burning fuel.
The problem is that it requires a lot of oxygen to make fuels burn cleanly and is very hard to do efficiently. What happens in the engine is that, like our stacking blocks analogy where we wanted a nice neat tower and because we rushed we ended up with a messy pile of blocks, some of the carbon atoms only find one oxygen atom to bond with and we get carbon monoxide, or perhaps some carbon atoms don’t find any oxygen atoms and become soot.
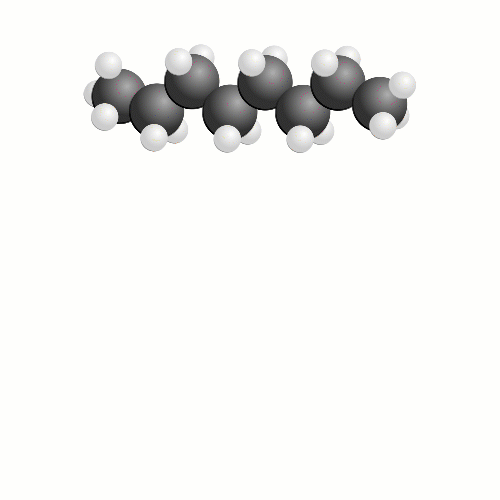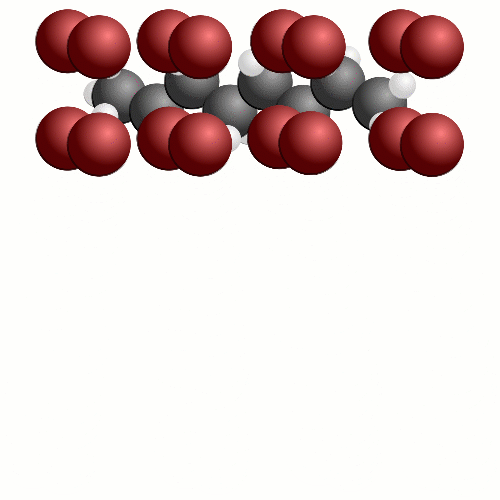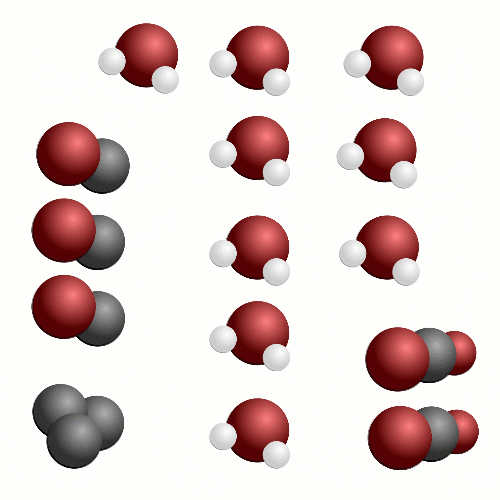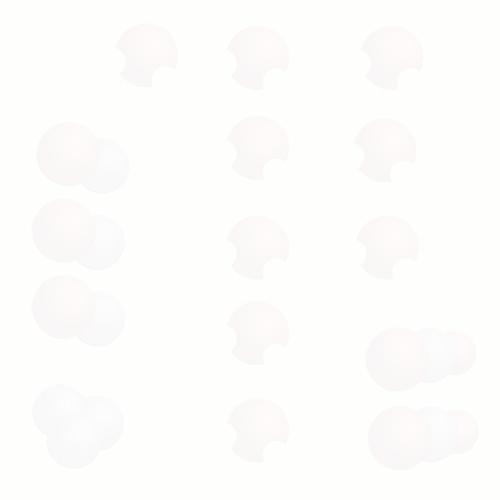
In this case, the entire octane molecule is reacting at once. It's a far more rapid reaction than the previous one we saw where the whole carbon chain was converted into carbon dioxide. This time, we cant get all the oxygen we need to the fuel at once. Instead of 12.5 oxygen molecules reaching the octane molecule we have 8. This means that some of the carbon atoms in octane will be turned into soot particles or carbon monoxide.
Even worse, there might be extra bits and pieces mixed in with your fuel that produce nitrogen based compounds when reacted with oxygen – nitrogen oxides. Nitrogen oxides, soot particles and carbon monoxide are bad for your health and also bad for the car – we are wasting energy that could be used to push the car along.
What’s different about an electric car?
 |
Our electric car doesn’t have fuel in the same way. Instead, the energy is stored up inside batteries. When we charge the batteries we fill them up with energy and that is used to power motors in the car to get us where we need to go.
The great thing about this is that you can make the energy to put into the battery however you like! Petrol and diesel can only ever come from millions of years of breaking down fossils deep underground but electricity can be generated from sunlight, wind, heat from underground or the waves and tides in the ocean. This means that we can make our electricity in a cleaner way without burning up fuels and producing greenhouse gases. |
Another advantage is that the gases that come out the back of car burning fuel are really not good for you! They say that a man who runs in front of a car will get tired but a man who runs behind a car will get exhausted. Electric vehicles don’t emit any gases while they’re being used so driving around the place that you live doesn’t damage the air or cause pollution – all the emissions happen wherever the electricity is produced which can be outside of town.
 Video
Video
You don’t just have to take my word for it – let’s hear what some of WMG’s battery experts think about electric vehicles.
 Conclusion
Conclusion
Electrifying vehicles is one way to reduce air pollution where you live and help us prevent dangerous climate change. The advantages of generating all the energy outside of town in a power plant - or even better a wind farm - and storing it up in batteries instead of burning fossil fuels are fantastic. The drawbacks are that at the moment there aren't enough places to charge your electric vehicle - not as convenient as driving to a petrol station - and they cost too much to buy. The research that is being done at places like the University of Warwick to develop new materials and technologies will help to make the cars more affordable, convenient and widespread. Hopefully we will see plenty more electric vehicles in the future!

 Introduction
Introduction  Focus
Focus  Video
Video  Conclusions
Conclusions  What's next?
What's next?  Authors
Authors 
