WMG News
Pushing the limits of battery research with nickel-rich chemistries
New research has shown that understanding how oxygen participates in energy storage is critical for developing higher energy density batteries, in a new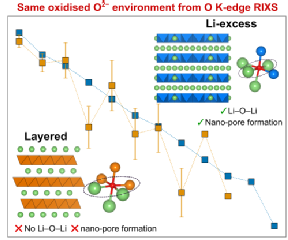 paper published by experts at WMG, at the University of Warwick.
paper published by experts at WMG, at the University of Warwick.
Using advanced X-ray techniques, researchers at WMG, together with the Faraday Institution's FutureCat consortium, have obtained new insights into the oxygen redox activity in conventional ni-rich cathodes, which will help to deliver improved electric vehicle performance.
Range anxiety is a key concern of many potential EV buyers, but range is steadily improving as battery technology and research evolves. The Faraday Institution’s Next Generation Lithium-Ion Cathode Materials project, FutureCat, aims to develop understanding of existing and newly discovered cathode chemistries to deliver improved EV performance, whilst considering sustainability.
Professor Louis Piper, from WMG at the University of Warwick, who led the research explained: “Transitioning to electrification requires integrating advanced materials science into battery processing to develop cheaper, safer, faster and better batteries, which is the focus of our research.”
The battery field is moving to increasing nickel contents in cathodes to meet the Government’s stringent EV 2030+ targets. These roadmaps assume successful strategies in material development to allow cathodes like W-LNO to operate at high voltages without degrading. This work provides the platform towards realising that goal by better understand the redox mechanisms (i.e., the reactions that enable charging/discharging the battery) at high voltage operation.
The study employed advanced x-ray characterisation techniques at the Diamond Light Source in Oxford and at WMG. The team at WMG utilised novel in-house x-ray absorption spectroscopy which enabled researchers to look at the electrode redox process of the battery cathodes after careful disassembly. Researchers were surprised to find that the oxidised oxygen species had the same characteristics as another group of Li-ion battery cathodes, Li-excess transition metal oxides. Reconciling how the same oxidised oxygen environment exists in both conventional and Li-excess cathodes is critical for unlocking how to develop the next generation of cathodes.
Professor Piper adds: “This work highlights how large-scale collaborative fundamental studies are needed even for supposedly ‘known’ systems.”
WMG will be continuing with further studies in this field, supported by the Faraday Institution, for the benefit of cathode battery manufacturers.
A link to the published article can be found here:
https://journals.aps.org/prxenergy/pdf/10.1103/PRXEnergy.2.013005
