Events @ Warwick Chemistry
Quantum Magnetism in Double Perovskites
On Friday 24th November, Dr Otto Mustonen (University of Birmingham) will deliver an invited talk titled “Quantum Magnetism in Double Perovskites”.
His talk will be held at 10am in L5. Everyone is welcome to attend, and if anyone wishes to meet with Otto then please get in touch with struan.simpson@warwick.ac.uk.
Abstract
Quantum materials have magnetic or electronic properties arising from a non-trivial quantum mechanic origin and potential applications from energy to quantum computing.1 Similarly, quantum magnets are magnetic materials with strong quantum effects and often unusual or exotic ground states. These typically arise in materials with low-dimensional interactions and low (“quantum”) spin on the magnetic cation such as S = 1/2 Cu2+ and V4+ or S = 1 Ni2+.
In this talk, I will present examples of quantum magnetism observed in the A2B’B’’O6 double perovskite family of compounds. The magnetic cations are typically located on the octahedral B-sites, which form a corner-sharing network. Sr2CuTeO6 and Sr2CuWO6 with Cu2+ are S = 1/2 square-lattice antiferromagnets, where the non-magnetic B’’-cation, either d10 Te6+ or d0 W6+, determines the type of magnetic order. Magnetic order is entirely suppressed in the solid solution Sr2CuTe1‑xWxO6 for x = 0.05-0.6, where the ground state has been proposed to be a disorder-induced spin liquid.2,3 Spin liquids are magnetic materials that do not order or freeze even at absolute zero. Sr2CuTe1-xWxO6 is the only spin liquid candidate with a square lattice, as was first predicted by Nobel Laureate Philip Anderson in 1987.4
4d and 5d transition metal compounds with significant spin-orbit coupling can also host exotic quantum states.5 In cubic d1 double perovskites, the spin (S =1/2) and orbital moment (L = 1) can become entangled to form Jeff = 3/2 states. The complex interactions between these can then give rise to the unusual magnetism. In the 4d1 Mo5+ compounds BaYMoO6 and Ba2LuMoO6, this leads to the formation of a valence bond glass state, where non-magnetic spin singlets are formed in a glassy manner.6
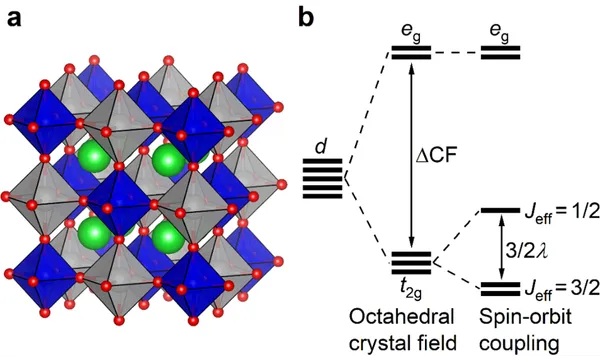
Figure 1. (a) The double perovskite structure of Ba2LuMoO6. Ba, Lu, Mo, and O are represented by the green, gray, blue, and red spheres, respectively. (b) Scheme of the orbital splitting for a 4d1 or a 5d1 cation.
1 R. Cava, N. de Leon, and W. Xie, Chem. Rev. 121, 2777 (2021).
2 O. Mustonen et al., Nat. Commun. 9, 1085 (2018).
3 O. Mustonen et al., Phys. Rev. B 98, 064411 (2018).
4 P. W. Anderson, Science 235, 1196 (1987).
5 W. Witczak-Krempa et al., Annu. Rev. Condens. Matter Phys. 5, 57 (2014).
6 O. H. J. Mustonen et al., npj Quantum Mater. 7, 74 (2022).
