News
High density of metal atoms in Si surface alloys essential for 2D supramolecular assembly
Costantini and collaborators report on the importance of metallic atoms in Si surface alloys for their use as effective substrates for 2D supramolecular self-assembly.
Scanning tunnelling microscopy is used to compare the assembly properties of terephthalic acid on two Bi-Si surface alloys with different metallic surface density. Results published in Surface Science show that, besides the absence of semiconductor dangling bonds, also a high density of Bi surface atoms is essential to smoothen the energy landscape experienced by adsorbed molecules and therefore promote their diffusion and assembly. More at http://www.sciencedirect.com/science/article/pii/S0039602811003177
Precious metal materials
Walton’s group, in collaboration with colleagues in the Department of Physics and at Johnson Matthey plc have a paper published this week in the RSC journal Chemical Science: this describes mild synthetic chemical routes to complex extended structures that contain the metal iridium in various oxidation states. This illustrates the scope for the discovery of new functional materials by exploration of novel reaction conditions and using the chemistry of lesser studied elements.
http://pubs.rsc.org/en/content/articlelanding/2011/sc/c1sc00192b/
Cisplatin as a protein crosslinker
Cisplatin is the most widely used anti-cancer drug, and the side-effects of cisplatin chemotherapy are largely due to side reactions of cisplatin with other molecules in the body, chiefly proteins. In a joint publication between the O'Connor group and the Sadler group, it's reported that the anticancer drug, cisplatin, can act as a protein crosslinker which is of use in the field of proteomics. Cisplatin has novel features as a protein crosslinker: 1) it's charged, so it improves the sensitivity of any modified peptides over non-reacted peptides, 2) it targets methionine and histidine primarily, which are residues that are not targetted by traditional crosslinkers, 3) it has an unusual isotopic pattern compared to typical peptides or peptide crosslinkers, which can be used as a signature to flag those peptides which are modified with platinum, 4) it has a fixed arm-length of about 4.5 Angstroms, but has the potential for addition of other chemical moities to extend the arm-length. This observation allows new kinds of proteomics that focuses on those proteins which are modified by cisplatin directly - thus potentially profiling all molecules which are directly responsible for the deleterious side-effects in chemotherapy. This paper has just been published in Analytical Chemistry: http://pubs.acs.org/doi/abs/10.1021/ac200861k
Challis group and collaborators feature on the cover of Chemistry & Biology
The group of prof. Challis together with their collaborators feature on the cover of Chemistry & Biology.
Through screening of Burkholderia cepaciacomplex bacteria, Mahenthiralingam et al. (pp. 665–677) identified one species (Burkholderia ambifaria) that produces potent polyketide antibiotics called enacyloxins, using an unusual hybrid, cis-AT/trans-AT polyketide synthase. The findings suggest that Burkholderiabacteria are a promising resource for the discovery of new antibiotics, with unusual production pathways and potent activity against drug-resistant bacteria. The cover shows a TLC plate on which Burkholderiaculture extracts were fractionated, illustrating the diversity of antimicrobial secondary metabolites. After overlaying this plate with agar seeded with the yeast Candida albicans, secondary metabolites with antimicrobial activity are revealed by the zones of clearing. Classical plate inhibition assays with Burkholderiastrains demonstrating antagonistic activity against a range of bacteria and fungi as well as the structure of enacyloxin IIa are also shown
To read the paper: http://dx.doi.org/10.1016/j.chembiol.2011.01.020
First wood-digesting enzyme found in bacteria could boost biofuel production
Researchers funded by the Biotechnology and Biological Sciences Research Council (BBSRC)-led Integrated Biorefining Research and Technology (IBTI) Club have identified an enzyme in bacteria which could be used to make biofuel production more efficient. The research is published in the 14 June issue of the American Chemical Society journal Biochemistry.
This research, carried out by teams at the Universities of Warwick and British Columbia, could make sustainable sources of biofuels, such as woody plants and the inedible parts of crops, more economically viable.
The researchers, who were also supported by the Engineering and Physical Sciences Research Council, have discovered an enzyme which is important in breaking down lignin, one of the components of the woody parts of plants. Lignin is important in making plants sturdy and rigid but, because it is difficult to break down, it makes extracting the energy-rich sugars used to produce bioethanol more difficult. Fast-growing woody plants and the inedible by-products of crops could both be valuable sources of biofuels but it is difficult to extract enough sugar from them for the process to be economically viable. Using an enzyme to break down lignin would allow more fuel to be produced from the same amount of plant mass.
The researchers identified the gene for breaking down lignin in a soil-living bacterium called Rhodococcus jostii. Although such enzymes have been found before in fungi, this is the first time that they have been identified in bacteria. The bacterium's genome has already been sequenced which means that it could be modified more easily to produce large amounts of the required enzyme. In addition, bacteria are quick and easy to grow, so this research raises the prospect of producing enzymes which can break down lignin on an industrial scale.
Professor Timothy Bugg, from the University of Warwick, who led the team, said "For biofuels to be a sustainable alternative to fossil fuels we need to extract the maximum possible energy available from plants. By raising the exciting possibility of being able to produce lignin-degrading enzymes from bacteria on an industrial scale this research could help unlock currently unattainable sources of biofuels.
"By making woody plants and the inedible by-products of crops economically viable the eventual hope is to be able to produce biofuels that don't compete with food production."
The team at Warwick have been collaborating with colleagues in Canada at the University of British Columbia who have been working to unravel the structure of the enzyme. They hope next to find similar enzymes in bacteria which live in very hot environments such as near volcanic vents. Enzymes in these bacteria have evolved to work best at high temperatures meaning they are ideally suited to be used in industrial processes.
Duncan Eggar, BBSRC Sustainable Bioenergy Champion, said: "Burning wood has long been a significant source of energy. Using modern bioscience we can use woody plants in more sophisticated ways to fuel our vehicles and to produce materials and industrial chemicals. This must all be done both ethically and sustainably. Work like this which develops conversion processes and improves efficiencies is vital."
ENDS
Notes to editors
This paper is available online here: http://pubs.acs.org/doi/abs/10.1021/bi101892z
Tweakable chiral magnetic materials
Magnetic interactions between metal atoms in a family of chiral materials respond to subtle changes in their organic chemistry. The work forms part of the PhD research by Lihong Li in the Scott group in Warwick Chemistry department. Read the article at Inorg. Chem. 2011
Light harvesting electron acceptor materials on cover of Advanced Energy Materials
Organic solar cells have potential as a low cost, easily producible renewable energy source. One of the key parameters determining the overall efficiency of the cells, the open-circuit voltage (VOC) generated, is critically dependent on the choice of active materials. Specifically, in a typical device where the current generation occurs at a hetero-interface between an electron donating material and an electron accepting material, the energy offset between the donor material's highest occupied molecular orbital (HOMO) and the acceptor material's lowest occupied molecular orbital (LUMO) dictates the magnitude of the generated VOC. Whilst an abundance of donor materials are known and reported, acceptor materials are typically limited to fullerene derivatives (especially C60) whose relatively low-lying LUMO energies limit the obtainable VOC. In the recent edition of "Advanced Energy Materials" (Vol. 1, Issue 3), the cover article by the Jones, Shipman and Hatton groups, along with collaborators at the University of Birmingham, reports a new synthetic design route to produce light harvesting electron acceptor materials which allow for high open-circuit voltages and power conversion efficiencies. By selective halogenation of the periphery of the organic framework of the traditional donor material boron subphthalocyanine chloride (SubPc), the HOMO and LUMO levels can be tuned such that the material can be used as an acceptor material in conjunction with an unsubstituted SubPc as the donor material. Optimisation of the number and type of halogen substituents such that efficient current generation takes place at the interface whilst maximising the interfacial energy offset results in a SubPc / Cl6-SubPc device which gives an exceptionally high VOC of over 1.3V and power conversion efficiencies approaching 3%. Further, as the unstable fullerene acceptor has been replaced by a subphthalocyanine derivative, a significant improvement in device stability is seen which is important for future commercialisation.
Front Cover (Design by Paul Sullivan): http://dx.doi.org/10.1002/aenm.201190011
Prof. Patrick Unwin made an ISE fellow
Professor Patrick Unwin, Department of Chemistry has been made a Fellow of the International Society of Electrochemistry (ISE). This award comes in recognition of the outstanding contributions Professor Unwin has made to the field of electrochemistry throughout his career.
Professor Unwin leads the Warwick Electrochemistry and Interfaces Group, who are known for developing innovative methods for functional imaging of surfaces and interfaces. They are currently in the process of developing a whole suite of new nanoscale electrochemical imaging methods with support from the European Research Council.
Professor Unwin said:
"I have been fortunate to have many good colleagues and fantastic students and postdocs in my 20 years at Warwick. They are great fun to work with and have contributed significantly to me winning this award."
About ISE
ISE is a large non-profit-making organisation set up to promote work done in the field of electrochemistry and provide an international community for those involved. It has around 2,300 members based in more than 60 countries worldwide.
Anticancer drug found to cause zinc deficiency
Cisplatin is responsible for abnormally low zinc levels in patients undergoing chemotherapy, say scientists in China and the UK.
Platinum-based compounds, like cisplatin, are the most widely used anticancer drugs in medicine. Research shows that up to 98 per cent of cisplatin binds to blood plasma proteins, particularly albumin. Until now, little has been known about the specific binding sites for platinum on albumin. 'Since albumin plays a major role in cisplatin metabolism, a better understanding of its interactions with albumin should lead to more effective use of the drug and avoidance or control of side effects,' says Peter Sadler from the University of Warwick, in the UK.
Cisplatin (structure in the middle) reacts with recombinant human albumin (rHA) (blue and green structures) to create a cisplatin-rHA adduct, which displaces zinc, causing a deficiency
Together with Fuyi Wang's team from the Chinese Academy of Sciences in Beijing, Sadler used mass spectroscopy techniques to reveal that cisplatin reacts with recombinant human albumin (rHA) to create a cisplatin-rHA adduct. The platinum occupies zinc binding sites on the albumin, displacing the zinc, which causes hypozincemia (lack of zinc for metabolic processes) and hyperzincuria (increased zinc in urine).
'Sadler's work nicely identifies coordination to two histidine amino acids, forming a cross-link between two peptides in the protein that are also implicated in Zn binding,' says Stephen Lippard, who studies the mechanism of cisplatin at the Massachusetts Institute of Technology, in the US. He adds that recognising this binding interaction paves the way for future studies. These studies could help determine whether the adduct facilitates transport to cancer cells or diverts cisplatin from its intended target, either clearing it from the body or leading to the toxic side effects, he explains.
Sadler says that platinum has another effect on albumin. One of albumin's roles is to transport fatty acids in the blood, but in the presence of platinum, the longer fatty acid chains are prevented from binding. 'Exciting challenges for future research include exploring the potential role of fatty acids in the allosteric regulation of binding both natural metal ions, such as zinc, and metallodrugs, such as platinum, to albumin,' says Sadler. He adds that the interactive effects of fatty acid and zinc binding to albumin have yet to be fully explored and that such understanding could have a major influence on therapeutic treatments in the future.
read the paper: http://dx.doi.org/10.1039/c1cc11627d
Prof. David Haddleton receives Royal Society Wolfson Research Merit Award
|
Professor David Haddleton, Department of Chemistry, has been awarded a Wolfson Research Merit Award by the Royal Society, the UK's national academy of science. This award aims to provide universities with additional support to attract to this country and retain respected scientists of outstanding achievement and potential. It is jointly funded by the Wolfson Foundation and the Department for Business, Innovation and Skills. Professor Haddleton will be working on a project entitled "Controlled polymerisation to new materials: Polymer therapeutics to oil recovery". He will take up his award by 1 May 2011. (see also The Royal Society |
An Electrode Design Rule for Organic Solar Cells Elucidated using Molecular Nanolayers
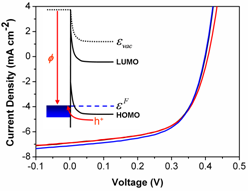
Publishing in Advanced Energy Materials the Hatton Group report an electrode design rule for organic solar cells which greatly simplifies an important aspect of device design: http://onlinelibrary.wiley.com/doi/10.1002/aenm.201100027/abstract
Using indium-tin oxide electrodes derivatized with silane nanolayers the performance of a model organic solar cell is correlated with the magnitude of the built-in positive space charge density in the critical region close to the window electrode. The results show that it is unnecessary to engineer the work function of the hole-extracting electrode in organic solar cells to match the ionisation potential of the adjacent organic semiconductor, rather only to ensure that the former exceeds the latter, thus simplifying an important aspect of device design. In addition, it is shown that molecular nanolayers at the window electrode surface are remarkably effective at retarding device degradation.
Nature Chemistry and PNAS for Challis group
Prof. Gregory Challis and his team bank two publications in the world leading journals Nature Chemistry and PNAS.
There is a constant need for new and improved drugs to combat infectious diseases, cancer, and other major life-threatening conditions. The recent development of genomics-guided approaches for novel natural product discovery has stimulated renewed interest in the search for natural product-based drugs. Genome sequence analysis of Streptomyces ambofaciens ATCC23877 has revealed numerous secondary metabolite biosynthetic gene clusters, including a giant type I modular polyketide synthase (PKS) gene cluster, which is composed of 25 genes (nine of which encode PKSs) and spans almost 150 kb, making it one of the largest polyketide biosynthetic gene clusters described to date. The metabolic product(s) of this gene cluster are unknown, and transcriptional analyses showed that it is not expressed under laboratory growth conditions. The constitutive expression of a regulatory gene within the cluster, encoding a protein that is similar to Large ATP binding of the LuxR (LAL) family proteins, triggered the expression of the biosynthetic genes. This led to the identification of four 51-membered glycosylated macrolides, named stambomycins A–D as metabolic products of the gene cluster. The structures of these compounds imply several interesting biosynthetic features, including incorporation of unusual extender units into the polyketide chain and in trans hydroxylation of the growing polyketide chain to provide the hydroxyl group for macrolide formation. Interestingly, the stambomycins possess promising antiproliferative activity against human cancer cell lines. Database searches identify genes encoding LAL regulators within numerous cryptic biosynthetic gene clusters in actinomycete genomes, suggesting that constitutive expression of such pathway-specific activators represents a powerful approach for novel bioactive natural product discovery. http://dx.doi.org/
Oxidative cyclizations, exemplified by the biosynthetic assembly of the penicillin nucleus from a tripeptide precursor, are arguably the most synthetically powerful implementation of C–H activation reactions in nature. Here, we show that Rieske oxygenase-like enzymes mediate regio- and stereodivergent oxidative cyclizations to form 10- and 12-membered carbocyclic rings in the key steps of the biosynthesis of the antibiotics streptorubin B and metacycloprodigiosin, respectively. These reactions represent the first examples of oxidative carbocyclizations catalysed by non-haem iron-dependent oxidases and define a novel type of catalytic activity for Rieske enzymes. A better understanding of how these enzymes achieve such remarkable regio- and stereocontrol in the functionalization of unactivated hydrocarbon chains will greatly facilitate the development of selective man-made C–H activation catalysts. http://dx.doi.org/10.1038/nchem.1024


