News
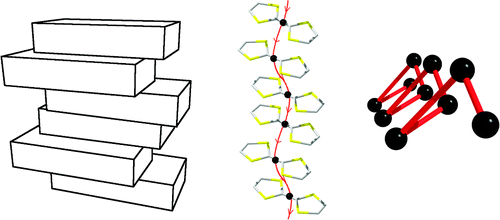
Corkscrew Conductors
A new family of semiconductors in which the electricity is transported through nanoscopic helices have been reported by the Scott group at Warwick. The work forms part of the PhD research by former Warwick Postgraduate Research Fellow
 , Dr Nikola Chmel
, Dr Nikola Chmel
 . Read the paper at Inorg. Chem. 2011
. Read the paper at Inorg. Chem. 2011
£150M for Warwick student infrastructure and proposed UG 2012 Fees of £9000
Warwick provides an extremely high quality teaching and learning environment that is valued, and highly sought after, by both students and staff. Warwick is already committed to further enhancements of that environment and we plan to invest around £150 million in our campus infrastructure between now and 2015.
Warwick students are taught by world-leading academics on a campus with a global reputation. Warwick is also consistently ranked as one of the UK’s top ten universities. We need to not just preserve that level of excellence, but to build on it and enhance it despite the reduction in funding that English universities are facing through the cuts to their teaching grants.
The University has held a number of discussions on how best to achieve those goals in a way that also enables the student finance system to promote widening participation. Those discussions have included meetings with: student representatives, Faculty Boards, and with Heads of Department. The University’s Senate and Council have now considered the feedback from those discussions, and have examined a proposal on fees, financial support and widening participation, at their meetings on 16th March (Senate) and 23rd March (Council).
Warwick's Senate and Council have now given their approval to a proposal for the University to set fees at £9,000 for Home and EU undergraduate students for 2012 entry.
The proposal, subject to agreement by the Office for Fair Access, would see students from state schools (or on full bursary schemes at Independent schools), whose family income falls under £25,000, receive a package of fee waivers and bursaries worth a total of up to £4,500, which is equal to half the cost of the standard fee. Around 1500 (19%) of Warwick’s current undergraduate student population falls within those terms.
Degree programmes specifically designed to offer opportunities that widen access, such as Warwick’s 2+2 degrees and part time degrees, would have a fee of £6,000 which may be further reduced by an additional fee waiver.
The proposal would also see Warwick invest around £9.6 million in new measures to further enhance the student experience.
Further details on these proposals will be available to undergraduate students for 2012 entry once they have been agreed with the Office for Fair Access in July 2011.
For further information please contact:
Peter Dunn, Head of Communications
University of Warwick 02476 523708
mobile 07767 655860 p.j.dunn@warwick.ac.uk
PR33 PJD 23rd March 2011
University of Warwick & partners awarded £1.4 million in national solar energy programme
The University of Warwick is a key part of a consortium that has just been awarded £1.4 Million by a new national programme designed to develop the next generation of solar energy harvesting technology.
The UK’s Technology Strategy Board and the Engineering and Physical Sciences Research Council has awarded the £1.4 million to a consortium that includes The University of Warwick; the companies Kurt Lesker, Asylum Research, New World Solar, and Molecular Solar, and Imperial College London. Together they will work on the development of Prototype High Efficiency Multi-Junction Organic Solar Cells.
Professor Tim Jones from the University of Warwick said:
“We are working with solar cells made from organic semiconductor materials which offer the prospect of very low cost manufacture of lightweight, flexible cells. They are made from sustainable materials and can be deployed as flexible sheets that could be used for a variety of applications including: a solar powered mobile phone charger that’s rolls up into a shape as small as the size of a pen, micro-lights that can be added to clothing, and a detachable sun-shade for automobile windscreens that powers a small integral fan to circulate air and cool the interior of the car when parked in direct sunlight.”
Peter Ballantyne from Molecular Solar, a spin-out company from the University of Warwick, which will be developing this new technology said:
“The low cost and flexibility of this new technology will lead to new applications that will further accelerate the growth of the solar power market, which has seen 40%/year growth over the last 10 years. Just one significant opportunity in consumer applications is the area of mobile phone chargers where over 1.3 billion units a year are produced.”
In total fifteen British businesses and seven universities will share £5 million of government funding from the UK’s Technology Strategy Board and the Engineering and Physical Sciences Research Council to enable them to research the use of novel nanoscale technologies to develop the next generation of solar energy harvesting.
Iain Gray, Chief Executive of the Technology Strategy Board said:
“These projects will help to position British businesses to exploit the growing global demand for solar energy harvesting technologies – and in the process help grow the British economy – while at the same time provide sustainable energy solutions for the UK. The projects are great examples of how to transfer commercially-focused research into the business community.”
David Delpy, Chief Executive of the Engineering and Physical Sciences Research Council said:
“This is the first example of Nanoscience research funding from the Research Councils being directly pulled through to application funding with the Technology Strategy Board via a stage-gated funding route. This approach actively supports economic growth whilst helping to solve one of society's greatest challenges.”
For further information please contact:
Professor Tm Jones University of Warwick Tel: +44 (0)2476 528265
Email: 

 t.s.jones@warwick.ac.uk
t.s.jones@warwick.ac.uk
Peter Dunn, Head of Communications
Communications Office, University House,
University of Warwick, Coventry, CV4 8UW, United Kingdom
email: p.j.dunn@warwick.ac.uk

Tel: +44 (0)24 76 523708 Mobile/Cell: +44 (0)7767 655860
PR34 25th March 2011
A Golden Window Electrode for Organic Solar Cells
The Hatton group together with Professor Tim Jones report the development of a rapid, solvent free method for the fabrication of large area gold films with a thickness of only 8 billionths of a metre, in Advanced Functional Materials: http://dx.doi.org/10.1002/adfm.201002021. These films combine high optical transparency and electrical conductivity with a remarkably low surface roughness and are exceptionally robust towards ultra-sonic agitation in a range of common solvents. By incorporating a random array of circular apertures into the films using microsphere lithography the team also show how the optical properties can be optimized for application as the transparent electrode in organic solar cells. These ultra-thin films are potentially widely applicable for a variety of applications, where stable, chemically well-defined, ultra-smooth transparent electrodes are required such as in the emerging fields of nanoelectronics and nanophotonics.


Bon Lab features on the cover of Polymer Chemistry
 |
A paper by PhD student Nicholas Ballard and associate professor Stefan Bon, previously selected by the Royal Society of Chemistry as a hot paper, features on the April 2011 cover of Polymer Chemistry. The paper entitled Hybrid biological spores wrapped in a mesh composed of interpenetrating polymer nanoparticles as "patchy" Pickering stabilizers describes a new method for the decoration of the intricate morphology of spore particles with polymer nanoparticles and investigates their behaviour at liquid–liquid interfaces. It is found that a large difference in the interfacial activity between spherical microspheres and the anisotropic particles synthesized exists, which is explained in terms of particle wettability. read the paper ; more on the BonLab |
Graphene oxide’s solubility disappears in the wash
|
Drs Rourke and Wilson’s team made their discovery when treating the graphene oxide with sodium hydroxide (NaOH) in an attempt to increase the usefulness of the oxygen containing functional groups believed to be bound to the graphene. Unfortunately it seemed to make things worse rather than better. Indeed at high enough concentrations of NaOH Dr Rourke was left with a black suspension. The Warwick led researchers recalled that it had been shown that oxidation debris adheres to carbon nanotubes but the weak nature of the connection of this oxidation debris to the carbon nanotubes meant that a wash with a base can simply remove the oxidative debris. Experiments showed that in that particular case oxidative debris was found to make up almost a quarter of the mass of the “oxidized carbon nanotubes”. The researchers felt a similar process maybe happening in the Graphene Oxide they were studying. The results may also help explain the inordinately high levels of oxygen people were claiming to find in graphene oxide. Chemists were already struggling to identify enough plausible carbon to oxygen bonds to accommodate the amounts of oxygen believed to form part of graphene oxide.
The remaining liquid was also dried to give a white powder that the Warwick researchers showed contained the “oxidative debris” or OD; the OD was shown to be made up exclusively of small, low molecular weight compounds (i.e. less than 100 atoms). The graphene oxide recovered from washing process formed about 64% of the mass of the “graphene oxide” at the start of the process. The recovered OD or oxidative debris formed at least 30% of the weight of the mass of the original “graphene oxide”. Drs Rourke and Wilson’s team believe this shows that much of the oxygen that was believed to be closely bonded to the carbon in the graphene oxide was actually not bonded at all but simply lying on top of the graphene sheets, loosely connected to them as “oxidative debris”. This oxidative debris contained a large quantity of oxygen that simply came out in the wash when the graphene oxide was treated with sodium hydroxide. This creates a significant proble Drs Rourke and Wilson say “Our results suggest that models for the structure of graphene oxide need revisiting. These results have important implications for the synthesis and application of chemically modified graphene particularly where direct covalent functionalization of the graphene lattice is required.” The paper entitled: The Real Graphene Oxide Revealed: Stripping the Oxidative Debris from the Graphene-like Sheets by Dr. Jonathan P. Rourke, Priyanka A. Pandey, Joseph J. Moore, Matthew Bates, Neil R Wilson (all of the University of Warwick), and Dr Ian A. Kinloch, Prof. Robert J. Young (The University of Manchester), has just been published in Angewandte Chemie DOI: 10.1002/anie.201007520. Notes for editors: The researchers thank Dave Hammond for help with thermogravimetric analysis (TGA), Lijiang Song for help with mass spectrometry, and Ajay Shukla for help with X-ray photoelectron spectroscopy (XPS), the Midlands Physics Alliance Graduate School for a scholarship. The TEM, TGA, and XPS instruments as well as the mass spectrometer used in this research were purchased with support from Advantage West Midlands (part funded by the European Regional Development Fund) as part of the Science City programme. For further information please contact: Dr Jonathan P. Rourke Peter Dunn, Head of Communications PR23 8th March 2011 |
Inorganic Materials book series
The fifth volume, Energy Materials, co-edited by Richard Walton, with Duncan Bruce (York) and Dermot O’Hare (Oxford), has been published this week by Wiley.
http://eu.wiley.com/WileyCDA/WileyTitle/productCd-0470997524.html

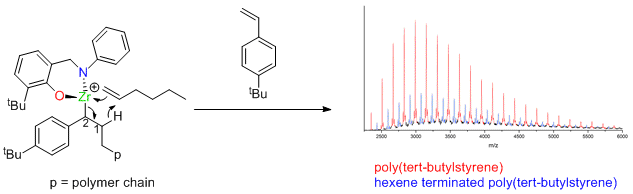
Zirconium catalysts not dead, just resting.
Dormant polymerization catalysts are given a rude awakening by Giles Theaker, Peter Scott and Warwick Chemistry Alumnus Colin Morton at Infineum. The results indicate how many old catalysts, thought to be dead, are just in a dormant state. The results published in the American Chemical Society journal Macromolecules describe a new mechanism for polymerization of styrenes.
JACS cover for Challis group
Prof. Greg Challis and his team together with Thomson and co-workers feature on this weeks cover of the Journal of the American Chemical Society. The absolute and relative stereochemistry of streptorubin B, a brightly colored prodiginine antibiotic, has been determined. Challis and co-workers utilized a mutant of Streptomyces coelicolor to conduct a mutasynthesis using enantioenriched deuterium-labeled biosynthetic precursors, while Thomson and co-workers developed an enantioselective total synthesis via a 10-membered pyrrolophane intermediate. See Challis and co-workers, p 1793, and Thomson and co-workers, p 1799. View the article.

Understanding Stimuli-Responsive Biomaterials
Matthew Gibson and collaborators investigate in detail the behaviour of stimuli-responsive polymer-protein conjugates in Polymer Chemistry. It is shown that at in vivo concentrations and when measured in blood rather than water, the behaviour of these materials deviates significantly from what is normally expected.
and collaborators investigate in detail the behaviour of stimuli-responsive polymer-protein conjugates in Polymer Chemistry. It is shown that at in vivo concentrations and when measured in blood rather than water, the behaviour of these materials deviates significantly from what is normally expected.
Konstantinos Bebis, Mathew W. Jones, David M. Haddleton and Matthew I. Gibson*. Polymer Chemistry, 2011, DOI: 10.1039/C0PY00408A
Read how the Bonlab armors polymer vesicles with colloids in JACS
|
The ability of some forms of plankton and bacteria to build an extra natural layer of nanoparticle-like armour has inspired chemists at the University of Warwick to devise a startlingly simple way to give drug bearing polymer vesicles (microscopic polymer based sacs of liquid) their own armoured protection. The Warwick researchers have been able to decorate these hollow structures with a variety of nanoparticles opening a new strategy in the design of vehicles for drug release, for example by giving the vesicle “stealth” capabilities which can avoid the body’s defences while releasing the drug. Advances in polymerisation have led to a surge in the creation of vesicles made from polymer molecules. Such vesicles have interesting chemical and physical properties which makes these hollow structures potential drug delivery vehicles. The University of Warwick team were convinced that even more strength, and interesting tailored properties, could be given to the vesicles if they could add an additional layer of colloidal armour made from a variety of nanoparticles.
“We took our inspiration from nature, in how it adds protection and mechanical strength in certain classes of cells and organisms. In addition to the mechanical strength provided by the cytoskeleton of the cell, plants, fungi, and certain bacteria have an additional cell wall as outermost boundary. Organisms that particularly attracted our interest were those with a cell wall composed of an armour of colloidal objects – for instance bacteria coated with S-layer proteins, or phytoplankton, such as the coccolithophorids, which have their own CaCO3-based nano-patterned colloidal armour” The Warwick researchers hit on a surprisingly simple and highly effective method of adding a range of different types of additional armour to the polymer based vesicles. One of those armour types was a highly regular packed layer of microscopic polystyrene balls. This configuration meant the researchers could design a vesicle which had an additional and precise permeable reinforced barrier for drug release, as a result of the crystalline-like ordered structure of the polystyrene balls. The researchers also succeeded in using the same technique to add a gelatine-like polymer to provide a “stealth” armour to shield vesicles from unwanted attention from the body’s immune system while it slowly released its drug treatment. This particular coating (a poly((ethyl acrylate)-co-(methacrylic acid)) hydrogel) absorbs so much surrounding water into its outer structure that it may be able to fool the body’s defence mechanism into believing it is in fact just water.
The research has just been published in a paper entitled Polymer Vesicles with a Colloidal Armor of Nanoparticles by Rong Chen, Daniel J. G. Pearce, Sara Fortuna, David L. Cheung, and Stefan A. F. Bon* Department of Chemistry, University of Warwick in the current Journal of the American Chemical Society http://dx.doi.org/10.1021/ja110359f Note for Editors: The cryo electron microscope used for the research have been funded by the Science City Research Alliance (SCRA) which is part of a larger investment by Advantage West Midlands and ERDF in the research infrastructure of the West Midlands region, which unites the University of Warwick and the University of Birmingham and the in a strategic research partnership – SCRA – formed under the Birmingham Science City initiative. Birmingham Science City, funded by Advantage West Midlands, is a region-wide partnership of public sector, businesses and the research base, which is facilitating the use of science and technology to improve the quality of life and prosperity of the West Midlands. For further information please contact: Peter Dunn, Head of Communications, Communications Office, PR10 31st January 2011 |
|
Dr Jósef Lewandowski joins Warwick Chemistry
We are delighted to announce that Dr Jósef Lewandowski will be joining us as Assistant Professor in Physical Chemistry from 1 September 2011.
Dr Lewandowski's research interests are primarily in the area of solid state NMR.
Jósef comes to us from the European Center for high Field NMR in Lyon, France where he is currently completing a postdoctoral stay with Prof Lyndon Emsley.






