News
Walton group's research highlighted on the front cover of Journal of Applied Crystallography
Recent work by the group of Richard Walton in collaboration with the group of Professor Pam Thomas of the Department of Physics at Warwick (Lethbridge et al. J. Appl. Crystallogr. 43 (2010) 168-175 ) has been selected as the cover image for the Journal of Applied Crystallography for 2011. The research involved the synthesis of usually large crystals of microporous zeolites, whose behaviour on heating and cooling was then examined using birefringence microscopy. This allowed new insights into the materials’ stability and structure as a function of temperature, including the migration of organic guest species through their structures.

Rachel O'Reilly and Andrew Dove guest edit themed issue of Polymer Chemistry
Rachel O'Reilly and Andrew Dove introduce the first themed issue of the journal Polymer Chemistry by the RSC (Royal Society of Chemistry) as guest Editors. The issue contains 2 reviews, 4 communications and 18 full papers of work by emerging investigators in the area of polymer chemistry. You can read this special issue of the journal here.
Scott Group detects chiral building blocks for new materials
Pairs of building blocks are shown using spectroscopic and electrochemical techniques to associate in solution before forming new types of charge transfer material relevant to the search for chiral conductors. The work forms part of the PhD research by former Warwick Postgraduate Research Fellow , Dr Nikola Chmel
, Dr Nikola Chmel .
.
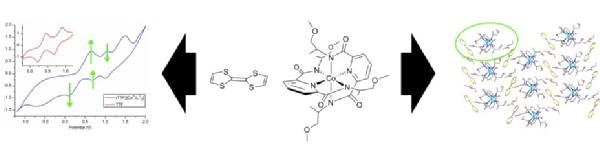
read the paper: http://dx.doi.org/10.1039/C0DT01184C
Shipman and Walsh groups report new method to quantify strength of hydrogen-bonds
Mike Shipman, Tiffany Walsh and co-workers have recently published a new method for detecting and quantifying noncovalent interactions. They have discovered that the rate of nitrogen inversion in aziridine derivatives is dependent on intramolecular interactions between attached functional groups. For example, the ortho-substituted pyridine undergoes faster inversion than its para-substituted analogue as a result of the formation of an intramolecular amide–pyridine (NH⋅⋅⋅N) hydrogen bond in the transition state (see graphic). Using simple NMR methods, it is possible to quantify the strength of these interactions in the transition state, and compare them with those predicted using computational methods. This work is expected to have applicability to a range of other important noncovalent interactions. It was conducted in collaboration with the Tucker group at the University of Birmingham.
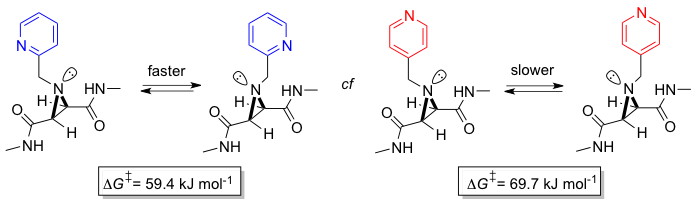
The paper is published in the 17 January 2011 issue of Angewandte Chemie.
http://onlinelibrary.wiley.com/doi/10.1002/anie.201005580/abstract
'Hot Article' In Chemical Communications from Walton Group
Richard Walton and his collaborators at the University of Versailles, France, have recently published a paper in Chemical Communications that reports the unusual hydration behaviour of a metal-organic framework material. The highly flexible structure undergoes a spontaneous expansion upon hydration to give a crystalline phase containing inifinite tubes of hydrogen-bonded water molecules. The hydration is completely reversible as shown by time-resolved X-ray diffraction. The work made use of high-resolution X-ray diffraction at the European Synchrotron Radiation Facility in Grenoble, and time-resolved powder X-ray diffraction in Warwick and forms part of a bigger project examining the flexibility of these materials as hosts for sorption and separation of a variety of molecules.
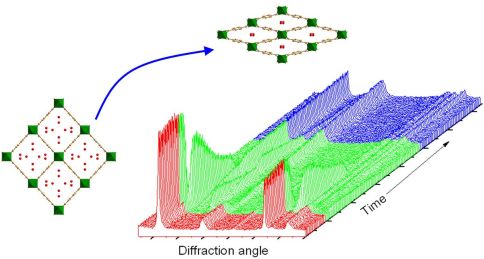
The paper is published in the 14 January 2011 issue of Chemical Communications and highlighted as a Hot Article by the publishers.
http://xlink.rsc.org/?doi=C0CC03882B
http://blogs.rsc.org/cc/2010/12/14/hot-article-round-up-for-november/
Platinum and Blue Light Combine to Combat Cancer
When it comes to health care blue lights, are usually most useful on the top of ambulances but now new research led by the University of Warwick has found a way to use blue light to activate what could be a highly potent platinum-based cancer treatment.
Research led by the University of Warwick, along with researchers from Ninewells Hospital Dundee, and the University of Edinburgh, have found a new light-activated platinum-based compound that is up to 80 times more powerful than other platinum-based anti-cancer drugs and which can use “light activation” to kill cancer cells in a much more targeted way than similar treatments.
The University of Warwick team had already found a platinum-based compound that they could activate with ultra-violet light but that narrow wave length of light would have limited its use. Their latest breakthrough has discovered a new platinum based compound known as trans,trans,trans-[Pt(N3)2(OH)2(py)2] that can be activated by normal visible blue, or even green, light. It is also stable and easy to work with, and it is water soluble so it can simply dissolve and be flushed out of the body after use.
The University of Warwick researchers passed the new compound to colleagues at Ninewells Hospital Dundee, who tested it on oesophageal cancer cells cultivated within lab equipment. Those tests show that once activated by blue light the compound was highly effective requiring a concentration of just 8.4 micro moles per litre to kill 50% of the cancer cells. The researchers are also beginning to examine the compound’s effectiveness against ovarian and liver cancer cells. Early results there are also excellent but that testing work is not yet complete.
Professor Peter Sadler, from the Department of Chemistry from University of Warwick, who led the research project, said:
“This compound could have a significant impact on the effectiveness of future cancer treatments. Light activation provides this compound’s massive toxic power and also allows treatment to be targeted much more accurately against cancer cells.”
“The special thing about our complex is that it is not only activated by ultra-violet light, but also by low doses of blue or green light. Light activation generates a powerful cytotoxic compound that has proven to be significantly more effective than treatments such as cisplatin.”
We believe that photoactivated platinum complexes will make it possible to treat cancers that have previously not reacted to chemotherapy with platinum complexes,” says Sadler. “Tumors that have developed resistance to conventional platinum drugs could respond to these complexes and with less side-effects.”
This research has been supported by the EPSRC, MRC, ERC and Science City (ERDF/AWM).
Note for editors: The research has just been published in Angewandte Chemie, under the title “A Potent Trans Diimine Platinum Anticancer Complex Photoactivated by Visible Light”. The authors are – Project leader Professor Peter Sadler, (University of Warwick) and Nicola J. Farrer, Julie A. Woods, Luca Salassa, Yao Zhao, Kim S. Robinson, Guy Clarkson, and Fiona S. Mackay.
For more information please contact:
Professor Peter Sadler
University of Warwick, Department of Chemistry
Tel: +44 (0)7913 944357
P.J.Sadler@warwick.ac.uk
Peter Dunn, Head of Communications, University of Warwick,
44 (0)24 76 523708
mobile/cell +44 (0)7767 655860
p.j.dunn@warwick.ac.uk
PR171 9th December 2010
Bonlab wraps biological spores in a polymer-based nanoparticle mesh
Nick Ballard and Stefan Bon report in Polymer Chemistry on a new method for the decoration of the intricate morphology of spore particles with polymer nanoparticles and investigate their behaviour at liquid–liquid interfaces. We found a large difference in the interfacial activity between spherical microspheres and the anisotropic particles synthesized here and describe this in terms of particle wettability. More on the bonlab; http://dx.doi.org/10.1039/C0PY00335B
Stefan Bon and collaborators fabricate Janus microspheres by "Sandwich" microcontact printing
Stefan Bon and collaborators report in Advanced Materials on A “sandwich” microcontact printing method. Monolayers of porous epoxy polymer microspheres made via microfluidics are transformed into Janus particles with distinct functionality on each face by reaction with amine functional fluorescent dyes, carbohydrates, and magnetic nanoparticles. http://dx.doi.org/10.1002/adma.201003564

Dixon group reports first atomic level structural data for smallest known oncoprotein
The E5 protein from bovine papllimavirus is a type II membrane protein and represents the smallest known oncoprotein. E5 functions via a unique mechanism, and in order to better understand this mechanism, Ann Dixon and previous lab members Gavin King and Joanne Oates, as well as current lab member Dharmesh Patel have used solution state NMR spectroscopy to obtain the first structural data for the E5 dimer in a membrane mimetic. These data were used to map the dimerization interface as well as calculate the free energy of dimerization for the E5 protein, in collaboration with Dr. Hugo van den Berg in the Mathematics Institute. The results agree very well with in vivo data, and further reinforce the importance of interactions in cell memebranes to biological function.
Mechanism of alkene hydroamination established by Peter Scott group and co-workers
A new class of highly active zirconium catalyst for the synthesis of chiral heterocycles from aminoalkenes is shown to operate via a Zr=N bonded (imido) species. Peter Scott and group members Laura Allan and Andrew Gott, in collaboration with David Fox and Guy Clarkson at Warwick, report in Journal of the American Chemical Society a detailed EPSRC-funded study which for the first time excludes the conventional Zr-N (amido) mechanism.
and group members Laura Allan and Andrew Gott, in collaboration with David Fox and Guy Clarkson at Warwick, report in Journal of the American Chemical Society a detailed EPSRC-funded study which for the first time excludes the conventional Zr-N (amido) mechanism.

Dr Manuela Tosin joins as assistant prof. of organic chemistry
Dr Manuela Tosin will be joining Warwick Chemistry as an Assistant Professor in Organic Chemistry from 1 November 2010. Dr Tosin's research interests are primarily in the area of the discovery and generation of new natural products. Manuela comes to Warwick Chemistry from the Department of Biochemistry, University of Cambridge where she worked with Dr Joe Spencer and Prof Peter Leadlay.

