News
Stefan Bon and Alessandro Troisi groups make the cover of Langmuir
Fascinating packing patterns of identical spherical and discotic objects on curved surfaces occur readily in nature and science. Examples include C60 fullerenes, 13-atom cuboctahedral metal clusters, and S-layer proteins on outer cell membranes. Numerous situations with surface-arranged objects of variable size also exist, such as the lenses on insect eyes and solid-stabilized emulsion droplets and bubbles. The cover image shows a collection of simulated packing patterns that can be obtained when spherical nanoparticles are assembled onto a central submicrometer-sized sphere. With the aid of Monte Carlo simulations, we are able to rationalize the experimental morphology and the nearest-neighbor distribution of the packing of nanosized silica particles on the surface of polystyrene latex particles fabricated by Pickering miniemulsion polymerization. We demonstrate that broadening of the nanoparticle size distribution has pronounced effects on the self-assembled equilibrium packing structures, with original 12-point dislocations or grain-boundary scars gradually fading out. For more information, see “Packing Patterns of Silica Nanoparticles on Surfaces of Armored Polystyrene Latex Particles” by Sara Fortuna, Catheline A. L. Colard, Alessandro Troisi, and Stefan A. F. Bon on pages 12399-12403 of this issue. View the cover.
Increased efficiency of small molecule photovoltaic cells by insertion of a MoO3 hole-extracting layer
I. Hancox, K. V. Chauhan, P. Sullivan, R. A. Hatton, A. Moshar, C. P. A. Mulcahy and T. S. Jones
We report a
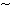 60% increase in open circuit voltage (Voc) and power conversion efficiency in a chloroaluminium phthalocyanine (ClAlPc)/fullerene (C60) planar heterojunction photovoltaic device after insertion of a MoO3 hole-extracting layer at the interface between the indium tin oxide (ITO) electrode and the ClAlPc donor layer, with an associated improvement in device stability. A similar improvement was observed in heterojunction devices based on mixed ClAlPc/C60 layers. We propose that the improvements in device performance are due to the pinning of the ITO Fermi level to the valance band of the MoO3 interlayer, where the latter is closely aligned with the highest occupied molecular orbital of ClAlPc.
60% increase in open circuit voltage (Voc) and power conversion efficiency in a chloroaluminium phthalocyanine (ClAlPc)/fullerene (C60) planar heterojunction photovoltaic device after insertion of a MoO3 hole-extracting layer at the interface between the indium tin oxide (ITO) electrode and the ClAlPc donor layer, with an associated improvement in device stability. A similar improvement was observed in heterojunction devices based on mixed ClAlPc/C60 layers. We propose that the improvements in device performance are due to the pinning of the ITO Fermi level to the valance band of the MoO3 interlayer, where the latter is closely aligned with the highest occupied molecular orbital of ClAlPc.

Unusual metals could forge new cancer drugs
The study, published in the Journal of Medicinal Chemistry, showed that a range of compounds containing the two transition metals Ruthenium and Osmium, which are found in the same part of the periodic table as precious metals like platinum and gold, cause significant cell death in ovarian and colon cancer cells.
The compounds were also effective against ovarian cancer cells which are resistant to the drug Cisplatin, the most successful transition metal drug, which contains the metal platinum.
Dr Patrick McGowan, one of the lead authors of the research from the School of Chemistry at the University of Leeds, explains: “Ruthenium and Osmium compounds are showing very high levels of activity against ovarian cancer, which is a significant step forward in the field of medicinal chemistry.
Sabine H. van Rijt, lead researcher in the laboratory of Professor Peter Sadler in the Department of Chemistry at the University of Warwick, said:
“Most interestingly, cancerous cells that have shown resistance to the most successful transition metal drug, Cisplatin, show a high death rate with these new compounds.”
Professor Sadler, at the University of Warwick, commented that he is “excited by the novel design features in these compounds which might enable activity to be switched on and off”.
Cisplatin was discovered in the 1970s and is one of the most effective cancer drugs on the market, with a 95% cure rate against testicular cancer. Since the success of Cisplatin, chemists all over the world have been trying to discover whether other transition metal compounds can be used to treat cancer.
In this type of anti cancer drug transition metal atoms bind to DNA molecules which trigger apoptosis, or programmed cell death, in the cancerous cells.
The study is a collaboration between the universities of Warwick and Leeds and was funded by the Engineering and Physical Sciences Research Council (EPSRC).
For more information please contact:
Dr Patrick McGowan at leeds +44 (0)113 343 6404, or email: p.c.mcgowan@leeds.ac.uk
Sabine H. van Rijt and Professor Peter Sadler from the University of Warwick are available for interview via Professor Peter Sadler +44 (0)24 76 523818 p.j.sadler@warwick.ac.uk
Jon Rourke's team shows a delicate balance between sp2 and sp3 C-H bond activation in a Pt(II) complex in JACS
2-tert-Butyl-6-(4-fluorophenyl)pyridine reacts with K2PtCl4 via the activation of an sp2 C−H bond to give a cyclometalated complex that contains a bifurcated agostic interaction. Rearrangement of this complex results in the activation of an sp3 C−H bond, and reaction eventually leads to a doubly cyclometalated complex where both sp2 and sp3 C−H bonds have been activated. Deuterium exchange studies show that a delicate balance exists between the two cyclometalations.
Stefan Bon's team and collaborators have the cover of Soft Matter with 2 papers in its issue
Stefan Bon's polymer colloids group and collaborators have two papers out in issue 20 of Soft Matter, one of which has made the cover. In the paper selected for the cover of Soft Matter  Bon, Keddie and coworkers show that when you add a small amount of a "soft" polymer latex armored with nanosized clay discs to a waterborne pressure sensitive adhesive (PSA), its performance respresented via the tack adhesion energy improves with 70%, as a direct result of the supracolloidal structure. A true synergistic effect was discovered showing for the first time that the clay-armored supracolloidal structure of the hybrid particles was essential to achieve a superior balance of viscoelastic bulk properties.
Bon, Keddie and coworkers show that when you add a small amount of a "soft" polymer latex armored with nanosized clay discs to a waterborne pressure sensitive adhesive (PSA), its performance respresented via the tack adhesion energy improves with 70%, as a direct result of the supracolloidal structure. A true synergistic effect was discovered showing for the first time that the clay-armored supracolloidal structure of the hybrid particles was essential to achieve a superior balance of viscoelastic bulk properties.
The second paper , a collaboration between David Cheung and Stefan Bon, describes the behaviour of "Janus"-type nanoparticles at liquid-liquid interfaces. Using simulations they demonstrate the adhesion and rotational behavior of a colloidal particle which has two "sides" each having a preferential affinity for one of the two bulk phases. Cheung and Bon demonstrate marked differences in behavior predicted by continueous theories.
, a collaboration between David Cheung and Stefan Bon, describes the behaviour of "Janus"-type nanoparticles at liquid-liquid interfaces. Using simulations they demonstrate the adhesion and rotational behavior of a colloidal particle which has two "sides" each having a preferential affinity for one of the two bulk phases. Cheung and Bon demonstrate marked differences in behavior predicted by continueous theories.
Electrospraying functional molecules onto solid surfaces as route to molecular coatings
Warwick shortlisted for University of the Year
Warwick has been shortlisted for 'University of the Year' in this year's Times Higher Education (THE) Awards.
Six universities have made the shortlist for the University of the Year award, which looks at the institution's performance in several areas such as research, teaching, access and business performance. Judges also look for specific evidence of particularly bold, imaginative and innovative initiatives that have advanced the University's reputation in the last year.
Warwick hosted a judging visit on Monday 7 September. THE editor Anne Mroz spent the day on campus, meeting a range of staff and visiting a number of departments and Centres including: the Capital Centre, Warwick Digital Laboratory, the new NMR facility and Warwick Arts Centre.
Warwick's Vice-Chancellor Professor Nigel Thrift said: "We are pleased to have been shortlisted for this award. Warwick has been consistently ranked as one of the UK's leading research universities and we provide a unique university experience for our students which they will remember and benefit from for years to come."
Ann Mroz, Editor, Times Higher Education, commented: "The Awards recognise the very best in higher education in the UK and we are delighted to announce that Warwick has made it onto the shortlist for University of the Year. Our senior advisory panel has been taken from all fields of academia and had to choose from hundreds of first-class entries – only the best made it through to our shortlist."
The Times Higher Education Awards, now in their fifth year, aim to recognise the cutting edge work undertaken by UK higher education institutions. Categories include Best Student Experience, Widening Participation Initiative of the Year, Outstanding Support for Overseas Students, Marketing Initiative of the Year, Outstanding Contribution to the Local Community and Outstanding Support for Disabled Students.
This year's winners will be announced at the Times Higher Education Awards ceremony, held at Grosvenor House Hotel, Park Lane, London, on 15 October 2009.
Open-cellular organic semiconductor thin films go smaller
Stefan Schumann, Stefan Bon, Ross Hatton and Tim Jones report in Chem.Commun: Vertical co-deposition of sub-100 nm polystyrene sphere templates with water-soluble small molecule or polymeric semiconductors, followed by solvent vapour assisted sphere removal, is shown to be an excellent method for generating porous large area organic semiconductor thin films with sub-100 nm open-cellular networks, with numerous potential applications in areas such as sensing and photovoltaics.
Small proteins cause a stir in transmembrane receptor binding and activation
Ann Dixon and collaborators report in Journal of Virology: The bovine papillomavirus E5 protein (BPV E5) is a 44-amino acid homodimeric transmembrane protein that binds directly to the transmembrane domain of the PDGF  receptor and induces ligand-independent receptor activation. Three specific features of BPV E5 are considered important for its ability to activate the PDGF
receptor and induces ligand-independent receptor activation. Three specific features of BPV E5 are considered important for its ability to activate the PDGF  receptor and transform mouse fibroblasts: a pair of C-terminal cysteines, a transmembrane glutamine, and a juxtamembrane aspartic acid. By using a new genetic technique to screen libraries expressing artificial transmembrane proteins for activators of the PDGF
receptor and transform mouse fibroblasts: a pair of C-terminal cysteines, a transmembrane glutamine, and a juxtamembrane aspartic acid. By using a new genetic technique to screen libraries expressing artificial transmembrane proteins for activators of the PDGF  receptor, we isolated much smaller proteins, from 32 to 36 residues, that lack all three of these features yet still dimerize non-covalently, specifically activate the PDGF
receptor, we isolated much smaller proteins, from 32 to 36 residues, that lack all three of these features yet still dimerize non-covalently, specifically activate the PDGF  receptor via its transmembrane domain, and transform cells efficiently. The primary amino acid sequence of BPV E5 is virtually unrecognizable in some of these proteins, which share as few as seven consecutive amino acids with the viral protein. Thus, small artificial proteins that bear little resemblance to a viral oncoprotein can nevertheless productively interact with the same cellular target. We speculate that similar cellular proteins may exist but have been overlooked due to their small size and hydrophobicity.
receptor via its transmembrane domain, and transform cells efficiently. The primary amino acid sequence of BPV E5 is virtually unrecognizable in some of these proteins, which share as few as seven consecutive amino acids with the viral protein. Thus, small artificial proteins that bear little resemblance to a viral oncoprotein can nevertheless productively interact with the same cellular target. We speculate that similar cellular proteins may exist but have been overlooked due to their small size and hydrophobicity.
Julie Macpherson in Nature Nanotechnology: Carbon nanotube tips for atomic force microscopy
The development of atomic force microscopy (AFM) over the past 20 years has had a major impact on materials science, surface science and various areas of biology, and it is now a routine imaging tool for the structural characterization of surfaces. The lateral resolution in AFM is governed by the shape of the tip and the geometry of the apex at the end of the tip. Conventional microfabrication routes result in pyramid-shaped tips, and the radius of curvature at the apex is typically less than 10 nm. As well as producing smaller tips, AFM researchers want to develop tips that last longer, provide faithful representations of complex surface topographies, and are mechanically non-invasive. Carbon nanotubes have demonstrated considerable potential as AFM tips but they are still not widely adopted. This review traces the history of carbon nanotube tips for AFM, the applications of these tips and research to improve their performance.
http://dx.doi.org/10.1038/nnano.2009.154
Icy Exposure Creates Armored Polymer High Tech Foams
Chemists and engineers at the University of Warwick have found that exposing particular mixtures of polymer particles and other materials to sudden freeze-drying can create a high-tech armored foam that could be used for a number of purposes, including a new range of low power room temperature gas sensors.
Freeze-drying has been used to create structured foams before, the first such experiments being with rubber in the 1940s with the ice crystals formed throughout this process acting as templates to form the porous foam structure.
However when trying to create particularly strong, stable polymer foam structures engineers and chemists today tend to rely on more complicated processes. The most straightforward of these methods is the so-called foaming or expanding process, which consists of introducing small discontinuities (for example by dispersing a compressed gas) into a soft polymer and then taking a further step to reinforce the cellular structure created upon polymerization or cooling.
The University of Warwick team’s new approach to fabricate polymer foams by “ice-templating” differs from previous strategies in that they use a special range of colloids (mixtures of small particles dispersed in water), with crucial differences in their hardness and size, as key building blocks. In particular they employ a blend of larger ‘‘soft’’ polymer latexes (with diameters in range of 200–500 nm) in conjunction with a range of much smaller ‘‘hard’’ nanoparticles such as silica (with diameters in range of 25–35 nm).
When such a mixture is exposed to freeze-drying the difference in diameters induces a concentration enrichment of the smaller harder particles in the mix near the wall of each growing ice crystal. This creates a cellular structured foam in just one step in which each cell is effective given an armored layer of the smaller, harder nanoparticles.
The Warwick researchers also found that by changing parameters, such as the nanoparticle/polymer latex ratios and concentrations, as well as the nanoparticle type, it was possible to fine-tune a certain the pore structure, and the overall porosity, of the polymer foams. The team were also able to employ various types of inorganic nanoparticles to create this instant freeze-dry foam armoring including: silica, Laponite clay, aluminium oxide, as well as small polystyrene latex particles.
The armored polymer foams have a range of applications but one of the most interesting could be a new range of room temperature low power gas sensors. The team increased the complexity of their mixture of colloids by the addition of a third colloidal component, carbon black particles with approximate diameters of 120 nm, which allowed them to produce an conductive foam 14% of the weight of which was carbon black particles.
Lead researcher Dr Stefan Bon from the University of Warwick’s Department of Chemistry said:
“This new process allows us to create interesting foam based nanocomposite materials which show promising results as gas sensors that can operate at room temperature and differ from traditional metal-oxide-based sensors. We know that existing chemical sensors formed from composites of carbon black particles and insulating polymers have been previously shown to form room-temperature (thus low-power) chemical sensors for detecting a range of volatile organic compounds. Now in one step we can place the same material in a high tech polymer foam to create a new range of gas-sensor devices. We believe these materials could become a new generation of sensing porous films.”
Notes to editors
The research paper entitled “Conducting Nanocomposite Polymer Foams from Ice-Crystal-Templated Assembly of Mixtures of Colloids” by Catheline A. L. Colard, Richard A. Cave, Nadia Grossiord, James A. Covington, and Stefan A. F. Bon (all from the University of Warwick) has just been published in Advanced Materials and features on the cover of issue 28. Adv.Mater. 2009, 21(28), 2894-2898.
Images are also available, contact Kelly Parkes-Harrison, Communications Officer, University of Warwick, 02476 57422, 07824 540863, k.e.parkes@warwick.ac.uk
Bruker and Warwick Chemistry announce collaboration in developing extreme performance mass spectrometry
COVENTRY, United Kingdom--(BUSINESS WIRE)--Bruker Daltonics announced today the establishment of a long-term collaborative programme for developing both applications and fundamental instrument technology in the area of extreme resolution mass spectrometry.
Building on over 14 years of experience in high performance mass spectrometry at the Department of Chemistry at Warwick, the University’s recent acquisition of both the new Bruker solariX™ 12 Tesla FTMS system and the maXis™ UHR-TOF system again puts the department at the forefront of technology for high performance mass spectrometry. At the core of the new instruments are dramatic improvements, up to an order of magnitude, in previous performance standards. These advances help address the University’s most challenging analyses including very complex mixtures in applications such as chemistry, medicinal discovery, protein interactions and petroleomics.
The collaboration is unusual in that it embraces not only topical applications innovation but also fundamental instrument development, the latter headed by Warwick Professor Peter O’Connor, who recently arrived from Boston University, and is one of the world’s most accomplished FTMS instrument development scientists. “We are very excited to be able to benefit from Peter’s ideas, and have arranged a technical fast-track for his developments to appear in our FTMS products,” commented Dr. Michael Schubert, Executive Vice President for R&D at Bruker Daltonics.
Professor Peter Sadler, Head of Chemistry at the University, whose research interests focus on metals in biology and medicine, the design and mechanism of action of metallodrugs, especially the role of proteins in metal-induced signal transduction said: “In my field state-of-the-art analysis of metal speciation holds the key to major breakthroughs in understanding both how metal ions control natural biological processes, and how metal complexes can be designed as novel therapeutic agents. Moreover, this new Bruker mass spec equipment, and the associated collaboration, will allow our newly established EPSRC Warwick Centre for Analytical Science to compete strongly at the forefront of the field.”
“We are delighted that Professors Sadler and O’Connor, who both have outstanding track records in the design and implementation of cutting-edge mass spectrometry, have chosen Bruker as a supplier and collaborative partner. It is especially gratifying to see real instrument development receiving such an energetic renewal in the UK,” commented Dr. Ian Sanders, Executive Vice President for Worldwide Sales and Marketing at Bruker Daltonics.
The solariX and maXis will be highlighted at the 18th International Mass Spectrometry Conference (www.imsc-bremen-2009.de) in Bremen, Germany from August 30 to September 4, 2009. For more information on IMSC 2009 and related Bruker Daltonics activities, please visit www.bdal.com/imsc.
ABOUT BRUKER DALTONICS
For more information about Bruker Daltonics and Bruker Corporation (NASDAQ: BRKR - News), please visit www.bdal.com and www.bruker.com.



