News
Giovanni Costantini transfers to Warwick Chemistry

 Giovanni Costantini transfers to Warwick Chemistry being the former group leader of the
Self-organized Growth and Quantum Structures Group in the
Nanoscale Science Department headed by
Prof. Klaus Kern at the
Max-Planck-Institute für Festkörperforschung in Stuttgart. He will take up his position full-time from January 2008.
Giovanni Costantini transfers to Warwick Chemistry being the former group leader of the
Self-organized Growth and Quantum Structures Group in the
Nanoscale Science Department headed by
Prof. Klaus Kern at the
Max-Planck-Institute für Festkörperforschung in Stuttgart. He will take up his position full-time from January 2008.
Recent work under Warwick Chemistry affiliation already includes:
Ordering of Dipeptide Chains on Cu Surfaces through 2D Cocrystallization
Wang, Y.; Lingenfelder, M.; Classen, T.; Costantini, G.; Kern, K.
J. Am. Chem. Soc.; (Communication); 2007; ASAP Article; DOI:
10.1021/ja075118v
Structure and Energetics of Diphenylalanine Self-Assembling on Cu(110)
Tomba, G.; Lingenfelder, M.; Costantini, G.; Kern, K.; Klappenberger,
F.; Barth, J. V.; Ciacchi, L. C.; De Vita, A.
J. Phys. Chem. A.; (Article); 2007; 111(49); 12740-12748.
Hydrogen and Coordination Bonding Supramolecular Structures of Trimesic
Acid on Cu(110)
Classen, T.; Lingenfelder, M.; Wang, Y.; Chopra, R.; Virojanadara, C.;
Starke, U.; Costantini, G.; Fratesi, G.; Fabris, S.; de Gironcoli, S.;
Baroni, S.; Haq, S.; Raval, R.; Kern, K.
J. Phys. Chem. A.; (Article); 2007; 111(49); 12589-12603.
Number one Review by Peter Sadler team
A review on Metals in Membranes by the Peter Sadler team was the most downloaded paper in Chem.Soc.Rev. in November 2007.
In this critical review we discuss recent advances in understanding the modes of interaction of metal ions with membrane proteins, including channels, pumps, transporters, ATP-binding cassette proteins, G-protein coupled receptors, kinases and respiratory enzymes. Such knowledge provides a basis for elucidating the mechanism of action of some classes of metallodrugs, and a stimulus for the further exploration of the coordination chemistry of metal ions in membranes. Such research offers promise for the discovery of new drugs with unusual modes of action. The article will be of interest to bioinorganic chemists, chemical biologists, biochemists, pharmacologists and medicinal chemists. (247 references)

Hot Paper in Langmuir by Stefan Bon Team
Pickering Miniemulsion Polymerization using Laponite Clay as a Stabilizer by Stefan Bon's research team is in the top 20 summer 2007 downloads in Langmuir.
Abstract:
Solid-stabilized, or Pickering, miniemulsion polymerizations using Laponite clay discs as stabilizer are investigated. Free radical polymerizations are carried out using a variety of hydrophobic monomers (i.e., styrene, lauryl (meth)acrylate, butyl (meth)acrylate, octyl acrylate, and 2-ethyl hexyl acrylate). Armored latexes, of which the surfaces of the particles are covered with clay discs, are obtained, as confirmed by scanning electron microscopy (FE-SEM) and atomic force microscopy (AFM). Overall polymerization kinetics of the Pickering miniemulsion polymerizations of styrene were investigated via gravimetry. Comparison with the bulk polymerization analogue clearly shows compartmentalization. Moreover, retardation effects up to intermediate monomer conversions are observed; they are more prominent for the smaller particles and are ascribed to the Laponite clay. A model is presented that allows for the prediction of the average particle size of the latexes produced as a function of the amounts of monomer and Pickering stabilizers used. It shows that under specific generic conditions the number of clay discs used correlates in a linear fashion with the total surface area of the latex particles. This is a direct result of the reversibility of the Laponite clay disc adhesion process under the emulsification conditions (i.e., sonication) used.
Hot Paper in Dalton Transactions' by Sadler team
106Ru radiolabelling of the antitumour complex [(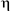 6-fluorene)Ru(en)Cl]PF6
6-fluorene)Ru(en)Cl]PF6
James D. Hoeschele, Abraha Habtemariam, Jeanette Muir and Peter J. Sadler, Dalton Trans., 2007
DOI: 10.1039/b706246j
The organometallic half-sandwich RuII arene anticancer complex [(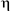 6-fluorene)Ru(en)Cl]PF6 (1) has been synthesized in high yield and purity on a micromole scale with incorporation of the
6-fluorene)Ru(en)Cl]PF6 (1) has been synthesized in high yield and purity on a micromole scale with incorporation of the 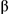 -emitting radioisotope 106Ru (half-life = 1.01 y) using a refined procedure involving conversion of RuCl3 into [(
-emitting radioisotope 106Ru (half-life = 1.01 y) using a refined procedure involving conversion of RuCl3 into [(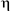 6-fluorene)RuCl2]2, and then [(
6-fluorene)RuCl2]2, and then [(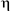 6-fluorene)Ru(CH3CN)2Cl]PF6 as intermediates. Distribution studies 0.25 h post i.v. injection of 106Ru-1 at a dose of 25 mg 1 kg–1 show that 106Ru is well distributed throughout the tissues of a rat. This appears to be the first report of the radiolabelling of a potential ruthenium antitumour agent for distribution/biological studies.
6-fluorene)Ru(CH3CN)2Cl]PF6 as intermediates. Distribution studies 0.25 h post i.v. injection of 106Ru-1 at a dose of 25 mg 1 kg–1 show that 106Ru is well distributed throughout the tissues of a rat. This appears to be the first report of the radiolabelling of a potential ruthenium antitumour agent for distribution/biological studies.

Tim Jones' research in top 10 downloads Journal of Materials Chemistry
Template directed synthesis of nanostructured phthalocyanine thin filmsby Tim Jones' team in top 10 downloads in J.Mater.Chem. (September 2007)
Warwick Chemistry tops EPSRC funding panel lists twice in a row!
Membranes do the trick
Researchers in the UK and New Zealand have shown that using a membrane could help catalysts operating in the same system work more efficiently.

The team, led by Paul Taylor at the University of Warwick and Andrew Livingston at Imperial College London, used a membrane to keep catalysts in environments where they work best.
Taylor explained that in a process where two or more catalytic steps are combined in one operation, called a tandem catalytic process, the catalysts normally have to compromise on their performance. This is because the same operating conditions are imposed on both catalysts. 'We use technological tricks to avoid the compromise,' he said, 'and allow the catalysts to operate under their respective optimum conditions, while in terms of the process they are in the same synthetic operation.'
- Paul Taylor, University of Warwick
The partnership involved collaboration between chemists interested in tandem catalysis and chemical engineers interested in membrane technology. Taylor explained that the collaboration resulted from effective networking with colleagues in industry interested in membrane separation.
Katherine Davies
Link to journal article
Towards a continuous dynamic kinetic resolution of 1-phenylethylamine using a membrane assisted, two vessel process
Chayaporn Roengpithya, Darrell A. Patterson, Andrew G. Livingston, Paul C. Taylor, Jacob L. Irwin and Mark R. Parrett, Chem. Commun., 2007, 3462
DOI: 10.1039/b709035h
Bacteria Genome Research Could Save Orchards and Assist Blood Transfusions
Nanotech Clay Armour Creates Fire Resistant Hard Wearing Latex Emulsion Paints
Researchers at the University of Warwick's Department of Chemistry have found a way of replacing the soap used to stabilize latex emulsion paints with nanotech sized clay armour that can create a much more hard wearing and fire resistant paint.
To date latex emulsion paints have relied on the addition of soaps or similar materials to overcome the polymer parts of the paint's aversion to water, stabilize the paint, and make it work. The University of Warwick chemistry researchers led by Dr Stefan Bon have found a simple way to individually coat the polymer particles used in such paints with a series of nanosized Laponite clay discs. The discs effectively create an armoured layer on the individual polymer latex particles in the paint. The clay discs are 1 nanometre thick by 25 nanometres in diameter (a nanometre is one billionth of a metre).
The Lapointe clay discs can be applied using current industrial paint manufacture equipment. They not only provides an alternative to soap but can also be used to make the paint much more hard wearing and fire resistant.
The process devised by the Warwick team can be used to create highly sensitive materials for sensors. The researchers can take closely packed sample of the armoured polymers and heat it to burn away the polymer cores of the armoured particles leaving just a network of nanotech sized connected hollow spheres. This gives a very large useful surface area in a very small space which is an ideal material to use to create compact but highly sensitive sensors.
Their research is in a paper enitled "Pickering Miniemulsion Polymerization Using Laponite Clay as a Stabilizer" by Stefan A. F. Bon and Patrick J. Colver and is published as the cover article in Langmuir. The ACS Journal of Surfaces and Colloids Vol. 23, Issue 16 July 31.
See: http://pubs.acs.org/cgi-bin/article.cgi/langd5/2007/23/i16/pdf/la701150q.pdf
For further information please contact:
Dr Stefan Bon
Associate Professor of Polymer Chemistry
Department of Chemistry. University of Warwick
Tel: 024 7657 4009 Email: S.Bon@warwick.ac.uk
Peter Dunn, Press and Media Relations Manager,
Communications Office, University House
University of Warwick, Coventry CV4 8UW
Tel: 024 76 523708 or 07767 655860
email: p.j.dunn@warwick.ac.uk
PR65 PJD 26th July 2007
Warwick Chemistry Researchers popular in Downloads
PhD student becomes vice-chair GRC Master Class
Warwick Chemistry popular on Youtube
Julie Macpherson's group Warwick ICAST on Youtube carbon nanotubes![]() is viewed more than 5,000 times. The short movie made over 8 months ago explains the research activities of Julie Macpherson's team from an educational viewpoint, focussing on nanoscience and carbon nanotubes. Warwick Chemistry also released a short movie on polymer colloids and supracolloidal chemistry by Stefan Bon's group, which has also proven popular with over 350 views in the last 2 months.
is viewed more than 5,000 times. The short movie made over 8 months ago explains the research activities of Julie Macpherson's team from an educational viewpoint, focussing on nanoscience and carbon nanotubes. Warwick Chemistry also released a short movie on polymer colloids and supracolloidal chemistry by Stefan Bon's group, which has also proven popular with over 350 views in the last 2 months.

