Publications
A complete up-to-date list of publications of Robert can be found on Google Scholar and full-texts might be available from the Warwick Research Active Portal (WRAP).
Size is not everything
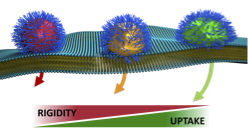 |
Stiffness matters in a size dependent manner, as found out by Dr Gurnani in a very enjoyable collaboration with Seb Perrier's group, and can now be read in "small" as Probing the effect of rigidity on the cellular uptake of core-shell nanoparticles: Stiffness effects are size dependent. The use of nanoparticles as vectors for the delivery of a wide range of biomedically relevant cargo is well established. Numerous studies have investigated the impact of size, shape, charge, and surface functionality of nanoparticles on mammalian cellular uptake. Rigidity, however, has been studied to a far lesser extent, and its effects are still unclear. Here, we systematically explore the importance of this property, and its interplay with particle size, using a library of core-shell spherical PEGylated nanoparticles synthesised by RAFT emulsion polymerisation. Rigidity of these particles was controlled by altering the intrinsic glass transition temperature (Tg) of their constituting polymers. Three different polymeric core rigidities were tested: hard, medium and soft using two particle sizes, 50 and 100 nm diameters. Cellular uptake studies indicated that softer particles are taken up faster and 3-fold more into mammalian cells compared to harder nanoparticles with the larger 100 nm particles. In addition, our study indicates major differences in the cellular uptake pathway, with harder particles being internalised through clathrin- and caveolae- mediated endocytosis as well as macropinocytosis, while softer particles were taken up by mainly caveolae- and non-receptor mediated endocytosis. However, our 50 nm derivatives did not show any appreciable differences in uptake efficiency suggesting that rigidity as a parameter in the biological regime might indeed be size dependent. |
Flow Rate Independent Multiscale Liquid Biopsy for Precision Oncology
Collaborative work with Jerome Charmet and Holosensor Medical Technology Ltd.
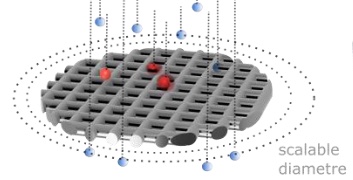 |
Immunoaffinity-based liquid biopsies of circulating tumour cells (CTCs) hold great promise for cancer management, but typically suffer from low throughput, relative complexity and post-processing limitations. Here we address these issues simultaneously by decoupling and independently optimising the nano-, micro- and macro-scales of a CTC enrichment device that is both simple to fabricate and operate. Our device achieved an 80% positive match in the identification of HER2+ breast cancer (n=26) compared to clinical standard FISH on solid biopsy. The results suggest that our approach, which overcomes major limitations previously associated with affinity-based liquid biopsies, could provide a versatile tool to improve cancer management. |
Renal transporter-mediated drug-biomarker interactions of the endogenous substrates creatinine and N1-methylnicotinamide: a PBPK modeling approach
Denise Türk from Thorsten Lehr's group at the University of Saarbrücken, Germany, led this work on PBPK modeling of biomarkers important for predicting drug-drug interactions mediated by kidney transporters. Obviously this is modulated by the circadian clock to some extend.
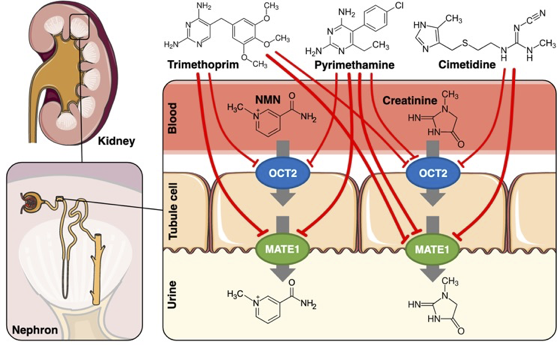
Drug-drug interactions (DDI) can lead to severe and unexpected side effects of medication because uptake, metabolism or excretion kinetics are significantly altered. For example, drug excretion in the kidney might be hampered because the relevant transporters are inhibited by a co-medication that is in the system at the same time. Thus, DDI studies are an important part of clinical investigations to determine the safety profile of novel and marketed drugs. However, testing multiple drugs in many different clinical scenarios is time-consuming and expensive. A possible improvement could be to study endogenous biomarkers that might predict DDI and facilitate better designed studies. Here, physiologically based pharmacokinetic (PBPK) modelling can help to characterise the behaviour of such biomarkers and establish the key components determining the kinetics.
Here, we developed PBPK models of the endogenous organic cation transporter (OCT) 2 and multidrug and toxin extrusion protein (MATE) 1 substrates creatinine and N1-methylnicotinamide (NMN). These performed well, i.e., aligned with the available clinical data and even took food and diurnal regulation into account. Then, the models were coupled with the previously built and evaluated models for the OCT2 and MATE1 inhibitors trimethoprim, pyrimethamine and cimetidine for DDI predictions. These models allowed us to predict previously unappreciated inhibition of NMN synthesis by trimethoprim that improved the fit to clinical data.
Thus, these whole-body PBPK models seem well suited to identify knowledge gaps and, ultimately, to facilitate investigations of renal transporter mediated DDIs during drug development.
#LittleTediousButReallyGreatResource
Impact of assessment frequency of patient-reported outcomes: an observational study using an eHealth platform in cancer patients
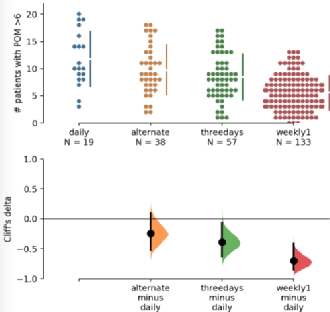 |
The evaluation of patient-reported outcomes (PRO) in cancer has proven relevant positive clinical impact on patients’ communication with healthcare professionals, decision-making for management, wellbeing and overall survival. However, the optimal frequency of PRO assessment has yet to be defined. Based on the assumption that more frequent sampling would enhance accuracy, we aimed at identifying the optimal sampling frequency that does not miss clinically relevant insight. Our analysis suggests that in patients receiving chemotherapy for advanced cancer, increasing the density of PRO collection enhances the accuracy of PRO assessment to a clinically meaningful extent. This is valid for both computation of averages symptom burden and for the recognition of episodes of severe symptom intensity. |
