Innovative design
|
Adaptive seamless design |
Subgroup selection |
|
Rare diseases and small populations |
|
|
|
Adaptive seamless design
In an adaptive seamless, or multi-arm-multi-stage, design a number of experimental treatments are compared simultaneously with a common control treatment, with data analysed at a series of interim analyses at which insufficiently effective treatments may be dropped from the study or the study may be stopped for efficacy if the result is already clear. Our work in this area has led to efficient and flexible design that control the familywise error rate and to methods for unbiased estimation in the final analysis of such studies. Our most recent work in this area has developed methodology when an early or surrogate endpoint is used for decision-making at interim analyses.
In addition to development of novel methodology, we have also been involved in a number of completed or ongoing clinical trials with adaptive seamless designs. These include a cluster-randomised trial in HIV prevention and individually randomised trials in surgery, physiotherapy and motor neurone disease.
Selected current and previous methodology projects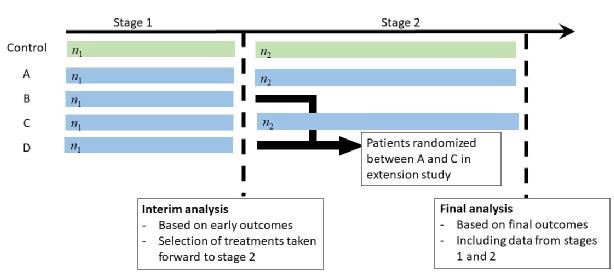
- MRC: Evaluation of Bayesian adaptive designs for Phase 3 effectiveness trials. 2017-2019 (PI: Gates, Birmingham; CoI: Stallard)
- MRC: Developing efficient perpetual platform trials to study multiple treatments and multiple biomarkers 2017-2020 (PI: Wason, Cambridge; CoI: Stallard)
- MRC: Using surrogate endpoints for decision-making in adaptive seamless designs. 2011-2015 (PI: Stallard)
Selected current and previous applied projects
- NIHR EME: Subacromial spacer for Tears Affecting Rotator cuff Tendons: a Randomised, Efficient, Adaptive Clinical Trial in Surgery (START:REACTS). 2018-2021 (PI: Metcalfe; CoI: Parsons)
- NIHR HTA: Physiotherapy Rehabilitation for Osteoporotic VErtebral fracture trial (PROVE). (PI: Barker, Oxford; CoI: Stallard).
Key papers
- Stallard, N. and Kimani, P. (2018) Uniformly minimum variance conditionally unbiased estimation in multi-arm multi-stage clinical trials. Biometrika, 105, 495-501, doi: 10.1093/biomet/asy004.
- Stallard, N., Kunz, C.U., Todd, S., Parsons, N., and Friede, T. (2015) Flexible selection of a single treatment incorporating short-term endpoint information in a phase II/III clinical trial. Statistics in Medicine, 33, 3104-3115.
- Stallard, N. (2010) A confirmatory seamless phase II/III clinical trial design incorporating short-term endpoint information. Statistics in Medicine, 29, 959-971.
- Stallard, N. and Todd, S. (2003) Sequential designs for phase III clinical trials incorporating treatment selection. Statistics in Medicine, 22, 689-703.
Subgroup selection
Closely related to multi-arm-multi-stage designs are ones in which is single experimental treatment is evaluated but it is anticipated that the treatment effect may differ in different parts of the population defined by some biomarker. Interim analyses can then be used to refine the population in which the treatment is evaluated in an adaptive enrichment design. We have developed methods for the design of trials of this type, and are currently working on methods for unbiased estimation in this setting, as well as on more general approaches for trials with multiple subgroups including umbrella and basket trial designs.
Selected current and previous methodology projects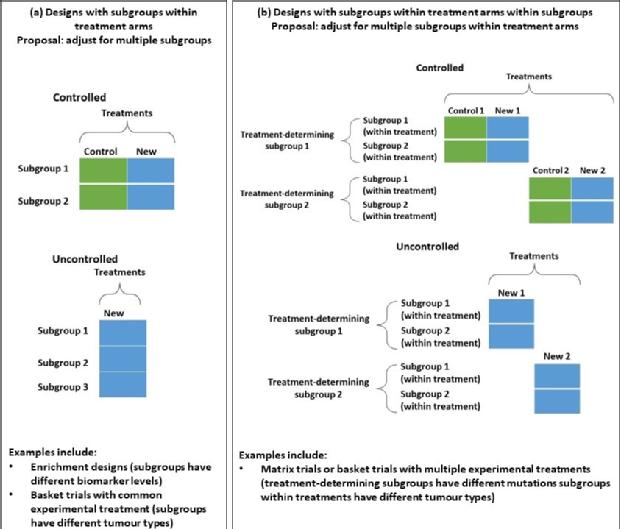
- MRC NIRG: Estimation of intervention effects for adaptive enrichment design RCTs that incorporate identification of predictive biomarkers. 2016-2019 (PI: Kimani)
- MRC: Using surrogate endpoints for decision-making in adaptive seamless designs. 2011-2015 (PI: Stallard)
Selected current and previous applied projects
- NIHR HTA: Optimal Personalised Treatment of early breast cancer using Multi-parameter Analysis (OPTIMA). (PI: Stein UCL; CoI: Stallard)
Key papers
- Stallard, N., Todd, S., Parashar, D., Kimani, P.K. and Renfro, L. Multiplicity in confirmatory clinical trials with master protocols. Annals of Oncology. To appear. DOI: 10.1093/annonc/mdz038
- Friede, T., Parsons, N. and Stallard, N. (2012) A conditional error function approach for subgroup selection in adaptive clinical trials. Statistics in Medicine, 31, 4309-4320.
- Kimani, P., Todd, S., Renfro, L. and Stallard, N. (2018) Point estimation following two-stage adaptive threshold enrichment clinical trials. Statistics in Medicine, 37, 3179-3196.
- Kimani, P.K., Todd, S. and Stallard, N. (2015) Estimation after subpopulation selection in adaptive seamless trials. Statistics in Medicine, 34, 2581-2601.
Rare diseases and small population
We are also interested in how innovative design methods can help to solve the particular challenges associated with clinical trials in rare diseases or other settings, such as stratified medicine, when the target population in small. We have used Bayesian decision theory to obtain designs that formally modify the level of evidence and sample size required so as to allow for the size of the population.
Selected current and previous methodology projects
- EU FP7: Innovative methodology for small populations research (InSPiRe) 2014-2017 (PI: Stallard)
Key papers
- Day, S., Jonker, A.H., Lau, l.P.L., Hilgers, R.-D., Irony, I., Larsson, K., Roes, K. and Stallard, N. (2018) Recommendations for the design of small population clinical trials. Orphanet Journal of Rare Diseases, 13: 195. doi: 10.1186/s13023-018-0931-2
- Stallard, N., Miller, F., Day, S., Hee, S.W., Madan, J., Zohar, S. and Posch, M. (2017) Determination of the optimal sample size for a clinical trial accounting for the population size. Biometrical Journal, 59, 609-625. doi: 10.1002/bimj.201500228
- Hilgers, R.-D., Roes, K. and Stallard, N. (2016) Directions for new developments on statistical design and analysis of small population group trials. Orphanet Journal of Rare Diseases, 11, 78. doi: 10.1186/s13023-016-0464-5
