Elemental Semiconductors
Silicon
Links
1. http://education.jlab.org/itselemental/ele014.html
2. http://www.radiochemistry.org/periodictable/elements/14.html
3. http://en.wikipedia.org/wiki/Silicon
C60
The C60 molecule is composed of 60 carbon atoms arranged in the shape of a soccer ball, made of twenty hexagons and twelve pentagons in a closed cage formation. Each atom is bonded to three others and is mixed sp2-sp3 hybridised. C60 behaves like electron-deficient alkenes and reacts with electron rich species. C60 is the most stable structure among fullerenes with a van der Waals diameter of about 1 nm.
Fullerenes can be produced in the residual soot obtained by applying an arc discharge in an inert atmosphere between two graphite electrodes. They play an important role in the development of molecular electronics. The electronic and physiochemical properties of cage molecules on the surfaces have been extensively studied by single molecule manipulation techniques and spectroscopy. C60 molecule has been studied to determine the qualitative and quantitative aspect of its conductivity properties. The nature of C60 has been concluded as semiconductor with a value of the energetic band gap varying according to local present interaction that might affect originally existing charges. Thus, for a neutral molecule 1.6 eV energy has been measured, 1.7 eV for a free molecule, while for molecules on metallic surfaces the gap is enlarge to 2.3 eV and even higher to 3.7 eV when the molecule is part of C60 bulk or island. When the molecule is surrounded by other C60 molecules, their molecular states overlap and the energy line broadens. If the molecule has non-covalent and weak interactions with its neighbours or with the substrate, the energetic gap tends to reduce.
Germanium and SiGe alloys
Silicon and germanium have similar proprieties such as belonging to the same group of the periodic table and being both indirect band gap semiconductors. Germanium has high bulk mobility values for electrons and holes, being this values two and four times higher than for silicon. These higher values imply a higher current with a smaller applied electric field leading to faster switching speeds. Also, the closer values for the mobility give rise to a more symmetrical CMOS. In the table below (reproduced from Dobbie) are stated some proprieties for germanium and silicon.
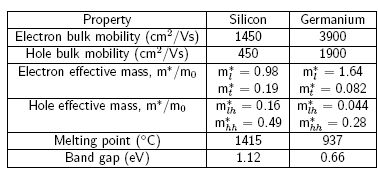
Germanium has an indirect band gap of 0.66 eV. Its band strucure is plotted below (reproduced from Seeger) aginst the wave number, k. As we can observe the band gap is located in the L minimum od the {111} direction. The conducion band is 8-fold degenerate at this point.

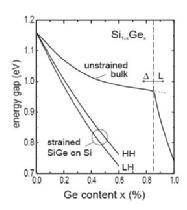
A way to lift the degeneracy is to is to grow germanium in silicon creating the alloy SiGe, usually designated by Si1-xGex, this is possible because, as already mentioned, silicon and germanium have the same crystal structure, diamond lattice. Strain in the alloy SiGe has a strong effect in the band structure, as x increases the indirect band gap decreases, also it is possible to observe an linear increase in the lattice parameter with x, as we can see it in the graph below (reproduced from Schaffer):
Links
1. http://en.wikipedia.org/wiki/Fullerene
2. http://www.nano-c.com/fullereneapp.html
3. http://www.ioffe.ru/IWFAC/2009/abstr/iwfac09__p010.pdf
Bibilography
1. Andrew DOBBIE “Investigation of the Electrical Properties of Si1-xGex channel pMOSFETs with High-k Dielectrics” PhD Thesis University of Warwick (2007).
2. F. Schäffler, High-mobility Si and Ge structures, Semicond. Sci. Technol. 12, 1515 (1997)
3. K. Seeger, Semiconductor Physics, An Introduction, 5th Edition, Springer series in Solid-State Sciences, 40 (1991).
