GaAs
Gallium arsenide (GaAs) is a compound semiconductor: a mixture of two elements, gallium and arsenic. Gallium is a by-product of the smelting of other metals, notably aluminum and zinc, and it is rarer than gold. Arsenic is not rare, but it is poisonous. Gallium arsenide has been developed for use in solar cells at about the same time that it's been developed for light-emitting diodes (LEDs), lasers, and other electronic devices that use light.
Basic Parameters
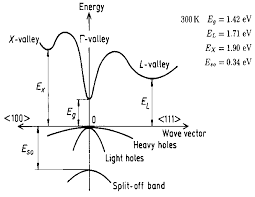
| Energy gap | 1.424 eV |
| Energy separation (EΓL) between Γ and L valleys | 0.29 eV |
| Energy separation (EΓX) between Γ and X valleys | 0.48 eV |
| Energy spin-orbital splitting | 0.34 eV |
| Intrinsic carrier concentration | 2.1·106 cm-3 |
| Intrinsic resistivity | 3.3·108 Ω·cm |
| Effective conduction band density of states | 4.7·1017 cm-3 |
| Effective valence band density of states | 9.0·1018 cm-3 |
Band structure and carrier concentration of GaAs300 K |
GaAs is a direct band gap semiconductor,which means that the minimum of the conduction band is directly over the maximum of the valance band.For that reason electrons need to change their energy not their momentum like in indirect band gap semiconductor such as Si.
Temperature dependence of the energy gap
Eg=1.519-5.405·10-4·T2/(T+204) (eV)
where T is temperatures in degrees K (0 < T < 103).
Temperature dependence of the energy difference between the top of the valence band and the bottom of the L-valley of the conduction band
EL=1.815-6.05·10-4·T2/(T+204) (eV)
Temperature dependence of the energy difference between the top of the valence band and the bottom of the X-valley of the conduction band
EL=1.981-4.60·10-4·T2/(T+204) (eV)
Intrinsic Carrier Concentration
ni =(Nc ·Nν )1/2exp(-Eg/(2kbT))
Effective density of states in the conduction band taking into account the nonparabolicity of the Γ-valley and contributions from the X and L-valleys
Nc= 8.63·1013·T3/2[1-1.9310-4·T-4.19·10-8·T2 +21·exp(-EΓL/(2kbT)) +44·exp(-EΓX/(2kbT)) (cm-3)
References
- http://www.osti.gov/bridge/servlets/purl/10189609.../10189609.pdf