Dean Keeble
 |
 |
| Contours | (Reciprical) Space |
What got you interested in this area of science?
We all learn at school that stuff - solids, liquids and gases alike - is made of atoms. Gases and liquids flow and compress and are just a bundle of atoms all floating around together, whereas solids always seemed a lot more interesting to me. How a solid behaves is governed only by the arrangements of the atoms, and so by changing that arrangement you can change how the material behaves! X-ray diffraction allows to look inside all manner of materials and measure how the atoms are arranged, with an accuracy of less than a ten thousandth of a billionth of a millimetre - which is pretty cool!
How is the work you are currently doing relevant to everyday life?
My work now is mostly concerned with trying to explain some interesting properties that we see in special materials that we call ferroelectric, by looking at the structure, which we can measure very accurately. Ferroelectrics are important for a wide range of uses, ranging from computer memory to ultrasound machines; from capacitors to electric barbeque lighters.
What do the images show?
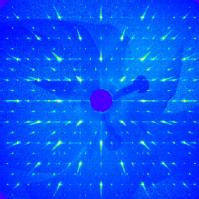
(Reciprocal) Space: A major plane of reciprocal space of Strontium Yttrium Manganate. The "Bragg" spots represent a direction of spatial periodicity in the crystal, and can reveal the exact arrangement of atoms that make up the structure. This image was acquired by illuminating a sub-millimetre piece of crystal with X-rays, and collecting the scattered photons. The faint lines of intensity between the main spots indicate that, on the nanometre scale, the crystal is locally disordered.
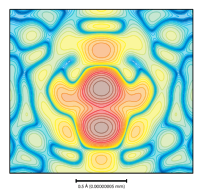
Contours:
X-ray diffraction experiments can be analysed to reveal where the electrons are in a material. Usually, there are a few areas with very high electron density: this is where the atoms are located. This contour plot shows exactly that, except in this case there are two peaks very close together. You'd never expect to find two atoms this close in a real material, so this experiment shows there is a degree of disorder, with different atoms taking each other's places in the structure. What we have measured here is an average, and we use this result to explain the physical properties we observe.
