Dr Jenny Wooldridge
Contact DetailsJenny now works at the National Physical Laboratory
National Physical Laboratory Hampton Road Teddington Middlesex TW11 0LW |
Ph.D. Research
After graduating from Bristol in 2003 I moved to Warwick to start work on cobalt oxide systems. This mainly consisted of single crystal growth (using the IR image furnaces we have here - not as easy as it looks....), sample characterisation (magnetic, heat capacity and resistivity measurements also done with the in-house equipment) and structure determination (x-ray and neutron scattering, models refined using GSAS and/or Fullprof).
My main research interest was NaxCoO2 (aka sodium cobalt oxide). It has an interesting magnetic phase diagram; two paramagnetic metallic phases are separated by a completely charge ordered phase at x = 0.5. The samples with higher sodium doping exhibit magnetic ordering below 22 K in the form of a spin density wave (SDW), the moments of which are ordered along the c-axis (see diagram below). At lower temperatures the magnetic ground state is further modified by the appearance of a weak ferromagnetic moment (also along c) and finally a pinning of the SDW onto the underlying Co lattice resulting in a 'glassy' ground state at around 4 K. These samples also show Na ordering transitions at ~340 K; this has been observed directly in a neutron diffraction experiment at the ILL. At the other end of the phase diagram (i.e. lower sodium concentrations) the crystal can superconduct with a critical temperature of 4.5 K provided sufficient water is intercalated between the CoO2 planes, increasing the two dimensionality in the system. Single crystal samples have been studied using neutron diffraction experiments to ascertain the nature of the water ordering within the NaxCoO2.yH2O superstructure. Click if you would like to know more about the magnetic ordering and here to find out what happens to the magnetism under pressure
I also worked on RBaCo2O5+d. It is a cobalt based oxygen deficient perovskite where R is a rare earth; the structure of the compound consists of layers of cobalt oxide that are separated by alternate layers of BaO and ROd, which in the d=0 phase results in the Co cations sitting in a pyramidal oxygen environment (see figure below). As more oxygen is doped into the system (in the ROd layer) the oxygen anions and vacant sites order into chains along the a axis so that the Co3+ ions have alternate octahedral (CoO6) and pyramidal (CoO5) coordination. The resulting crystal field splitting is of similar energy to Hund's coupling constant and consequently there are three possible spin states for the cobalt, which vary as a function of oxidation and temperature. This system has competing AFM-FM phases at temperatures lower than 260 K and a MI transition at 360 K; neutron diffraction experiments designed to look at the magnetic structure were problematic (!) and so x-ray experiments using circular dichroism are planned for the near future.
| NaxCoO2 | RBaCoO2O5+d |
|---|---|
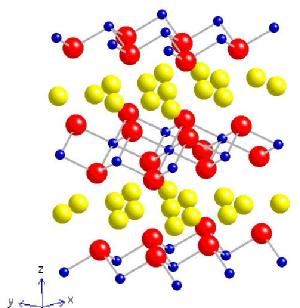 |
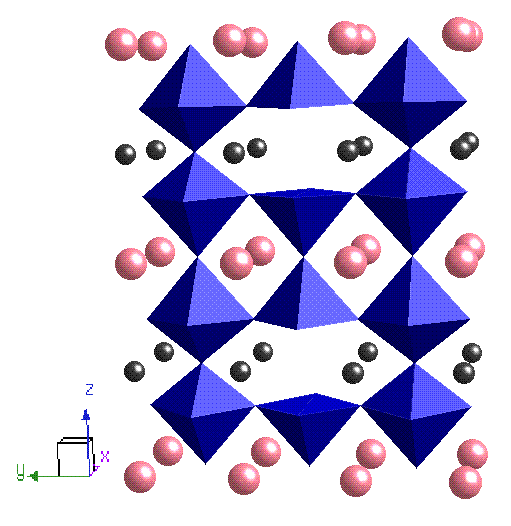 |
| Blue = Co, Red = O, Yellow = Na | Pink = Ba, Black = R, Blue = CoO5(6) octahedra |
After graduation in December 2006, Jenny moved to London, where she now works as a Research Scientist at the National Physcal Laboratory.
Back to the Former Members of the Group
Thesis Abstract
Superconductivity and magnetism in sodium cobaltate oxyhydrate
Thesis
Superconductivity and magnetism in sodium cobaltate oxyhydrate
