Battery Research
Strength of Materials - Dr Jiaqi Duan
Meet the Academic
What advice would you give to a young people aspiring to get in to a STEM Career?
“If you are interested in what is happening in the world around you, then just go for it. There are lots of opportunities available.”
To learn more about Mona and her journey:
Download the transcriptLink opens in a new window
Bringing Science to Life
How do batteries work?
The electricity is generated through the chemical reactions of lithium in the battery. Lithium-ion batteries are charged and discharged by lithium ions moving between the negative and positive electrodes. Every single layer of a Li-ion battery is essential, and it cannot function when one of the components is missing. For example, a thin aluminium foil is used to hold the frame of the cathode. This is called (positive) current collector. Cathode is an important layer in battery as it defines the characteristics such as energy capacity, voltage range and lifetime. Similar to cathode, anode is made up of a copper film (current collector) coated with active material. The active material in anode is responsible for enabling the electric current to flow the external circuit when allowing the reversible emission of lithium ions released from the cathode.
The movement of lithium-ions happen through electrolyte and the movement of electrons happen through wires. The electrolyte in fact serves as a medium for the movement of ions between cathode and anode. It is made up of high ionic conductivity material (salts, solvents, and additives). When cathode and anode determine the performance of cell, the separator determines the safety of the battery and work as a physical barrier between the cathode and anode. It plays an important role in preventing the direct flow of electrons and only lets the passage of ions through the internal microscopic pores.
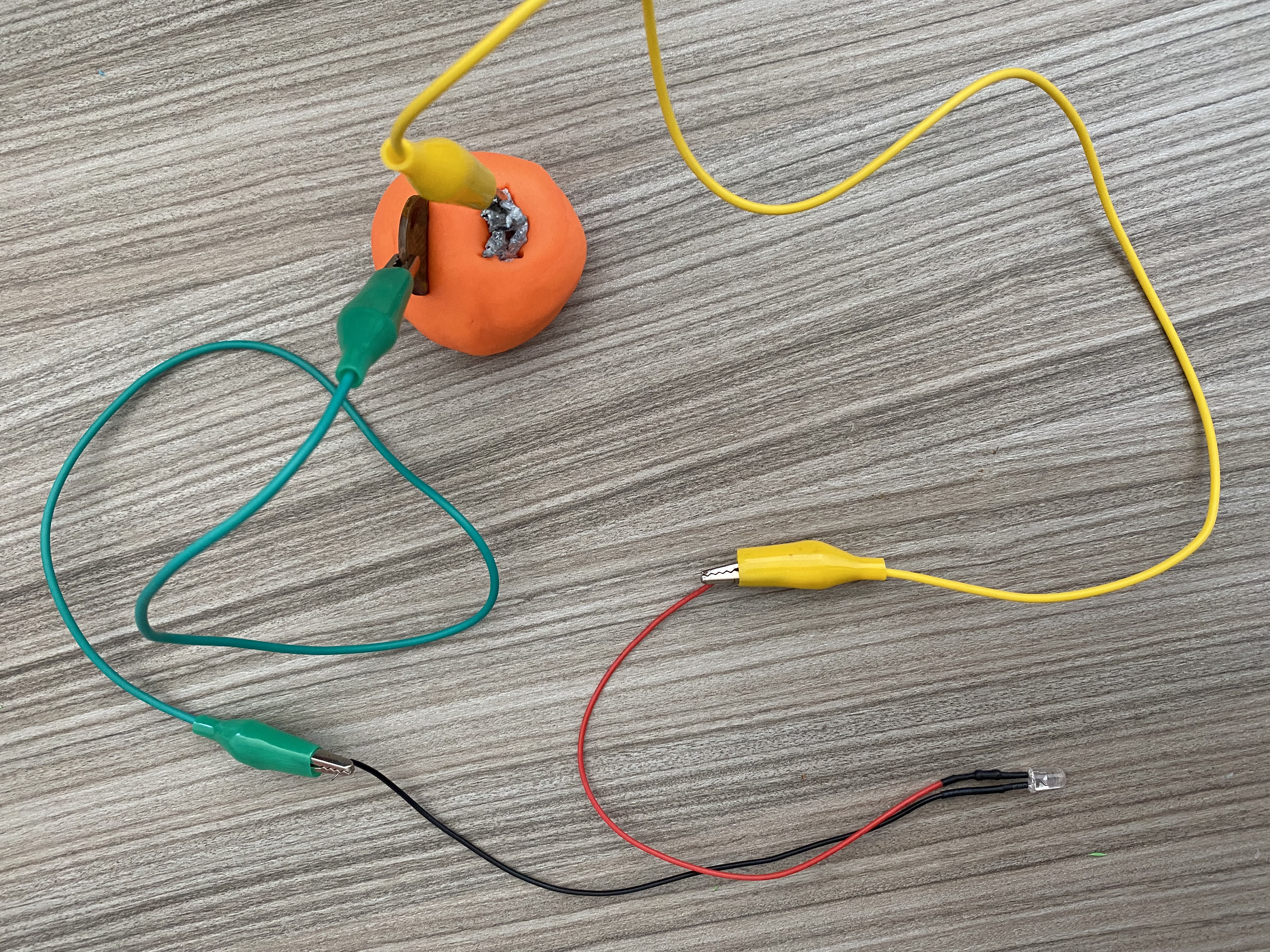
How to make to your own battery
Materials required:
- Copper coins
- Galvanised nail
- LED
- Crocodile clips and wires
- Volt meter
- Playdough (can also use lemons/potatoes/salt water)
- 3V battery
Step by step instructions:

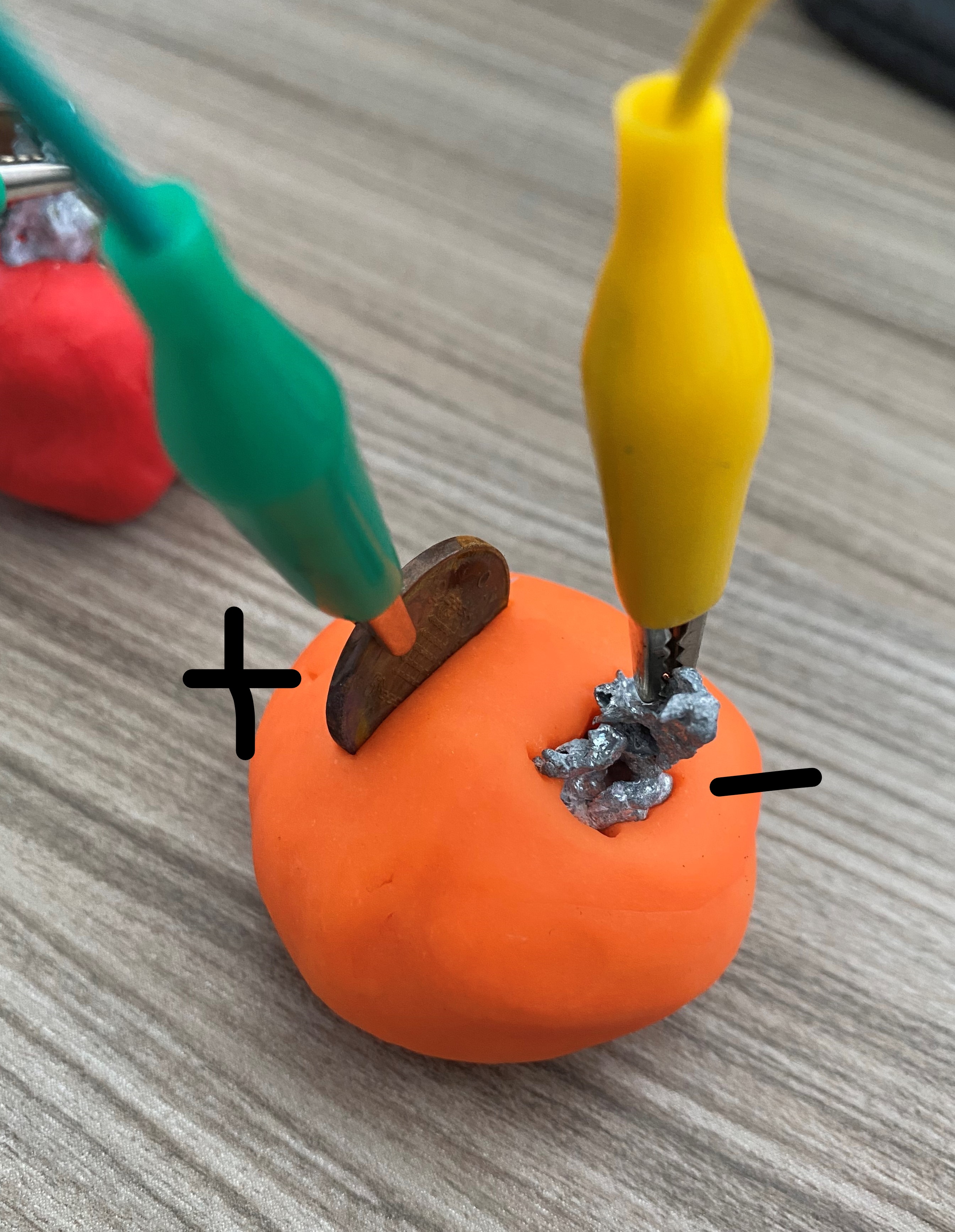

To explore this concept further:
- What happens if you use more than one LED?
- How does the size of the playdough affect the voltage measured?
- Try to make a battery in the same with other materials such as lemons and see how it differs.
Bringing Science to Life resources were created by interns: Seorin Park and Laura Lotkowska.

