WMG News
Battery life for wearable electronic devices could be improved with design considerations to stress asymmetry clues in cylindrical battery cell formats
Researchers in WMG and the Department of Physics at the University of Warwick have found that asymmetric stresses within electrodes used in certain wearable electronic devices provides an important clue as to how to improve the durability and lifespan of these batteries.
Batteries for medical applications and wearable devices continue to evolve in size and shape, with miniaturisation of Li-ion technologies becoming increasingly popular. However, as the size of the battery shrinks, the fabrication process for composite electrodes and the use of liquid electrolyte is becoming a processing challenge for microfabrication using conventional approaches.
 Lithium cobalt oxide LiCoO2 (LCO) has remained a common choice of cathode for these small formats due to its high voltage platform and energy density. However, following the initial reported performance benefits of LCO, it is known that LCO cells have large impedance issues due to the growth of high surface layer resistance and charge transfer resistance. This can affect how efficiently the battery charges and discharges. There are also ethical and health considerations around the use of the element cobalt. The increasing impedance was thought to be attributable to the growth of a surface layer on both the anode (solid electrolyte interface, SEI) and cathode (cathode electrolyte interface, CEI) due to the reaction between the electrodes and the electrolyte.
Lithium cobalt oxide LiCoO2 (LCO) has remained a common choice of cathode for these small formats due to its high voltage platform and energy density. However, following the initial reported performance benefits of LCO, it is known that LCO cells have large impedance issues due to the growth of high surface layer resistance and charge transfer resistance. This can affect how efficiently the battery charges and discharges. There are also ethical and health considerations around the use of the element cobalt. The increasing impedance was thought to be attributable to the growth of a surface layer on both the anode (solid electrolyte interface, SEI) and cathode (cathode electrolyte interface, CEI) due to the reaction between the electrodes and the electrolyte.
However, in the paper “Ageing analysis and asymmetric stress considerations for small format cylindrical cells for wearable electronic devices” published recently in the Journal of Power Sources, the University of Warwick’s WMG and Physics department researchers disassembled these cells. They have found that and the condition of the cathode and anode varied greatly after 500 cycles, as a function of which side of the current collector it was on.
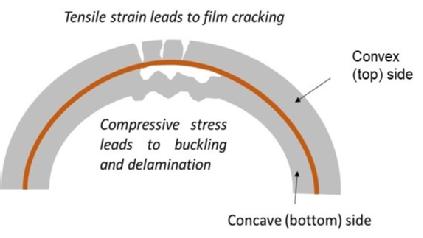
The inward facing cathode (under compression) when rolled into a jelly-roll, develops significant signs of coating delamination from the aluminium foil. On the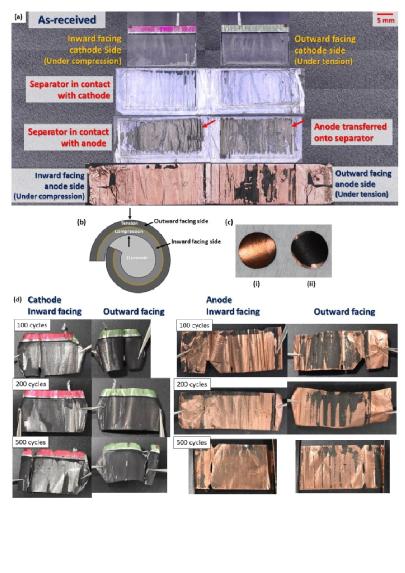 outward facing cathode side (under tension), however, only a partial delamination was evident and the coating was transferred unto the separator. By contrast, severe delamination was observed on both sides of the anode coating. The inward facing anode side (under compression) showed almost no coating still adherent to the copper foil, compared to the outward facing anode side (under tension). Likewise, the delaminated coating had become adhered to the separator during operation.
outward facing cathode side (under tension), however, only a partial delamination was evident and the coating was transferred unto the separator. By contrast, severe delamination was observed on both sides of the anode coating. The inward facing anode side (under compression) showed almost no coating still adherent to the copper foil, compared to the outward facing anode side (under tension). Likewise, the delaminated coating had become adhered to the separator during operation.
Dr Mel Loveridge from WMG, University of Warwick comments:
“It is interesting to note that, for both the cathode and the anode, the delamination is more severe on the electrode coating side that would have been subjected to compression stress, rather than tensile strain. This can be further explained by considering the asymmetric forces in place on either side of double side coated electrodes.”
The research team also carried out electrochemical testing, X-ray photoelectron spectroscopy (XPS), X-ray computed tomography (XCT) and scanning electron microscopy SEM), to reveal the battery’s structural features and changes. They found that it maintains 82% cell capacity after 500 continuous charging and discharging, after which it shows severe delamination due to high bending stress exerted on the cell components. However this seemingly has minimum impact on the electrochemical performance if the coating is sufficiently compressed in the jelly roll with a good electrical contact. After ageing, the surface layers continue to grow, with more LiF found on the cathode and anode.
Their research opens up exciting areas in battery manufacturing to address winding issues for cylindrical cells (especially miniaturised formats). For example, highlighting the need to understand whether there is merit in varying the coating properties on each side of double-sided coating for wound cylindrical cells, in order to improve the mechanical resilience of coatings that have asymmetric stresses exerted on them.
ENDS
25 AUGUST 2020
NOTES TO EDITORS:
Paper available to view: https://www.sciencedirect.com/science/article/abs/pii/S0378775320309307?via%3Dihub
High-res images available at:
https://warwick.ac.uk/services/communications/medialibrary/images/july_2020/wearable_image.jpg
Caption: An image demonstrating the stresses to anodes and cathodes after cycles.
Credit: WMG, University of Warwick
https://warwick.ac.uk/services/communications/medialibrary/images/july_2020/mel_l.jpg
Caption: A diagram of how tensile strain leads to the film cracking
Credit: WMG, University of Warwick
The full research team on the paper were:
Ageing analysis and asymmetric stress considerations for small format cylindrical cells for wearable electronic devices C.C. Tan, Marc Walker, Guillaume Remy, Nadia Kourra, Faduma Maddar, Shaun Dixon, Mark Williams and Mel Loveridge.
For further information please contact:
Alice Scott
Media Relations Manager – Science
University of Warwick
Tel: +44 (0) 7920 531 221
E-mail: alice.j.scott@warwick.ac.uk
How you charge your mobile phone could compromise its battery lifespan
Researchers at WMG at the University of Warwick have found that use of inductive charging, whilst highly convenient, risks depleting the life of mobile phones using typical LIBs (Lithium-ion batteries)
Consumers and manufacturers have ramped up their interest in this convenient charging technology, abandoning fiddling with plugs and cables in a favour of just setting the phone directly on a charging base.
Standardisation of charging stations, and inclusion of inductive charging coils in many new smartphones has led to rapidly increasing adoption of the technology. In 2017, 15 automobile models announced the inclusion of consoles within vehicles for inductively charging consumer electronic devices, such as smartphones – and at a much larger scale, many are considering it for charging electric vehicle batteries.
Inductive charging enables a power source to transmit energy across an air gap, without the use of connecting wire but one of the main issues with this mode of charging is the amount of unwanted and potentially damaging heat that can be generated. There are several sources of heat generation associated with any inductive charging system – in both the charger and the device being charged. This additional heating is made worse by the fact that the device and the charging base are in close physical contact, any heat generated in one device may be transferred to the other by simple thermal conduction and convection.
In a smartphone, the power receiving coil is close to the back cover of the phone (which is usually electrically non-conductive) and packaging constraints necessitate placement of the phone’s battery and power electronics in close proximity, with limited opportunities to dissipate heat generated in the phone, or shield the phone from heat generated by the charger. It has been well-documented that batteries age more quickly when stored at elevated temperatures and that exposure to higher temperatures can thus significantly influence the state-of-health (SoH) of batteries over their useful lifetime.
The rule of thumb (or more technically the Arrhenuis equation) is that for most chemical reactions, the reaction rate doubles with each 10 °C rise in temperature. In a battery, the reactions which can occur include the accelerated growth rate of passivating films (a thin inert coating making the surface underneath unreactive) on the cell’s electrodes. This occurs by way of cell redox reactions, which irreversibly increase the internal resistance of the cell, ultimately resulting in performance degradation and failure. A lithium ion battery dwelling above 30 °C is typically considered to be at elevated temperature exposing the battery to risk of a shortened useful life.
Guidelines issued by battery manufacturers also specify that the upper operational temperature range of their products should not surpass the 50−60 °C range to avoid gas generation and catastrophic failure.
These facts led WMG researchers to carry out experiments comparing the temperature rises in normal battery charging by wire with inductive charging. However the WMG were even more interested in inductive charging when the consumer misaligns the phone on the charging base. To compensate for poor alignment of the phone and the charger, inductive charging systems typically increase the transmitter power and/or adjust their operating frequency, which incurs further efficiency losses and increases heat generation.
This misalignment can be a very common occurrence as the actual position of the receiving antenna in the phone is not always intuitive or obvious to the consumer using the phone. The WMG research team therefore also tested phone charging with deliberate misalignment of transmitter and receiver coils.
All three charging methods (wire, aligned inductive and misaligned inductive) were tested with simultaneous charging and thermal imaging over time to generate temperature maps to help quantify the heating effects. The results of those experiments have been published in the journal ACS Energy Letters in an article entitled “Temperature Considerations for Charging Li-Ion Batteries: Inductive versus Mains Charging Modes for Portable Electronic Devices.”
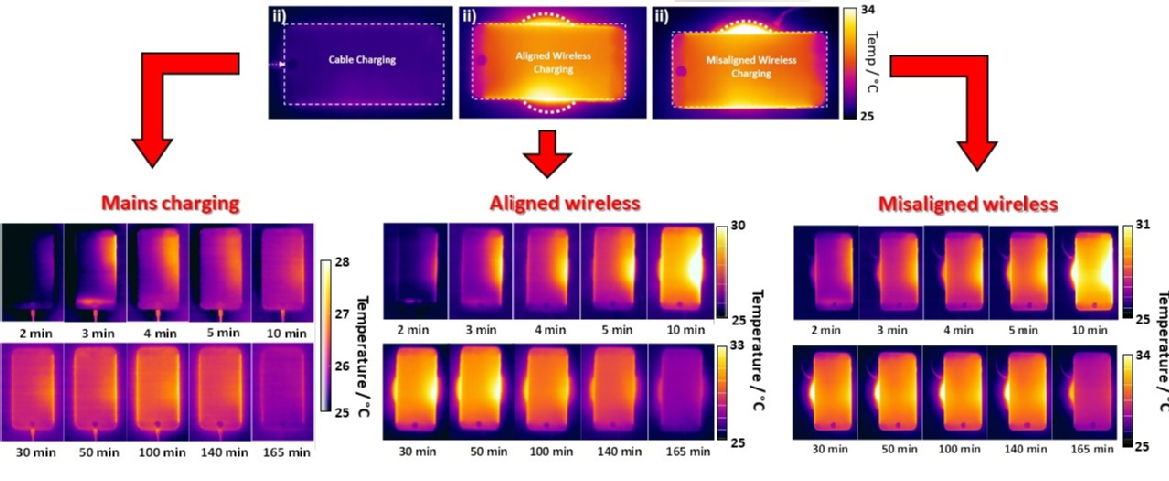 The graphics with this press release illustrates three modes of charging, based on (a) AC mains charging (cable charging) and inductive charging when coils are (b) aligned and (c) misaligned. Panels i and ii show a realistic view of the charging modes with a snapshot of the thermal maps of the phone after 50 min of charging. Regardless of the mode of charging, the right edge of the phone showed a higher rate of increase in temperature than other areas of the phone and remained higher throughout the charging process. A CT scan of the phone showed that this hotspot is where the motherboard is located In the case of the phone charged with conventional mains power, the maximum average temperature reached within 3 hours of charging did not exceed 27 °C.
The graphics with this press release illustrates three modes of charging, based on (a) AC mains charging (cable charging) and inductive charging when coils are (b) aligned and (c) misaligned. Panels i and ii show a realistic view of the charging modes with a snapshot of the thermal maps of the phone after 50 min of charging. Regardless of the mode of charging, the right edge of the phone showed a higher rate of increase in temperature than other areas of the phone and remained higher throughout the charging process. A CT scan of the phone showed that this hotspot is where the motherboard is located In the case of the phone charged with conventional mains power, the maximum average temperature reached within 3 hours of charging did not exceed 27 °C.
In contrast this for the phone charged by aligned inductive charging, the temperature peaked at 30.5 °C but gradually reduced for the latter half of the charging period. This is similar to the maximum average temperature observed during misaligned inductive charging.
In the case of misaligned inductive charging, the peak temperature was of similar magnitude (30.5 °C) but this temperature was reached sooner and persisted for much longer at this level (125 minutes versus 55 minutes for properly aligned charging).
Also noteworthy was the fact that the maximum input power to the charging base was greater in the test where the phone was misaligned (11W) than the well-aligned phone (9.5 W). This is due to the charging system increasing the transmitter power under misalignment in order to maintain target input power to the device. The maximum average temperature of the charging base while charging under misalignment reached 35.3 °C, two degrees higher than the temperature detected when the phone was aligned, which achieved 33 °C. This is symptomatic of deterioration in system efficiency, with additional heat generation attributable to power electronics losses and eddy currents.
The researchers do note that future approaches to inductive charging design can diminish these transfer losses, and thus reduce heating, by using ultrathin coils, higher frequencies, and optimized drive electronics to provide chargers and receivers that are compact and more efficient and can be integrated into mobile devices or batteries with minimal change.
In conclusion, the research team found that inductive charging, whilst convenient, will likely lead to a reduction in the life of the mobile phone battery. For many users, this degradation may be an acceptable price for the convenience of charging, but for those wishing to eke out the longest life from their phone, cable charging is still recommended.
ENDS
26 JUNE 2019
NOTES FOR EDITORS
While one specific model of mobile phone was used to conduct the tests the issues raised obviously apply to all phones or portable devices now or in the future that seek to use inductive charging.
High-res image available at: https://warwick.ac.uk/services/communications/medialibrary/images/june2019/iphone_charging_mode_2.jpg
Credit: WMG, University of Warwick
Paper available to view at: https://pubs.acs.org/doi/10.1021/acsenergylett.9b00663
List of Authors (all WMG) include:
Melanie. J. Loveridge
Chaou C. Tan
Faduma M. Maddar
Guillame Remy
Mike Abbott
Shaun Dixon
Richard McMahon
Ollie Curnick
Mark Ellis
Mike Lain
Anup Barai
Mark Amor-Segan
Rohit Bhagat
Dave Greenwood
New report says UK electric vehicle battery industry could be worth £2.7 billion per year for UK chemical companies
 A new report published today Monday 25th June 2018 shows that UK companies are well-placed to supply valuable materials needed for batteries to be built in UK – a potential £2.7 billion per year business opportunity. The report commissioned by WMG at the University of Warwick, was launched to the Chemical Industry Association at the Chemistry Growth Partnership meeting in London, chaired by Steve Foots, Chief Executive of Croda, and attended by Richard Harrington MP.
A new report published today Monday 25th June 2018 shows that UK companies are well-placed to supply valuable materials needed for batteries to be built in UK – a potential £2.7 billion per year business opportunity. The report commissioned by WMG at the University of Warwick, was launched to the Chemical Industry Association at the Chemistry Growth Partnership meeting in London, chaired by Steve Foots, Chief Executive of Croda, and attended by Richard Harrington MP.
The research underpinning the report brought together experts and data from the automotive battery industry and chemicals industry, working in the context of the UK’s Industrial Strategy, points to a large UK battery manufacturing industry opportunity. The report was funded by EPSRC, commissioned and managed by WMG at the University of Warwick acting in their role as the Advanced Propulsion Centre Electrical Energy Storage Spoke, and delivered in partnership with E4tech. WMG’s Professor David Greenwood, one of the report’s authors said:
“This report details a massive opportunity to grow a UK battery chemicals industry and related supply chain. The UK’s Industrial Strategy identified battery development and manufacture as one of the four initial Grand Challenges to coalesce industrial activity upon high growth opportunities. Battery pack manufacturing for electric vehicles (EVs) will logically take place close to the point of vehicle assembly since packs are hard to transport. This in turn implies that the battery cells which make up the packs will best be manufactured in (or close to) the UK. This could also mitigate the loss of vehicle engine production.”
“However for cell production to occur in the UK, the supply chains of chemicals would need to be reconfigured, since most cell production and chemicals supply is currently in Asia. Whilst such components could be imported, to capture the most value cell production and the related chemical and process equipment supply would need to come from UK suppliers.”
RESOLVE prototypes shape future of electric urban commuting
 The future of daily urban commuting could be small, lightweight Electric L-category Vehicles (ELVs). A cost effective, energy efficient and comfortable alternative to traditional cars in cities, is at the heart of the €6.92m RESOLVE project, which included WMG at the University of Warwick.
The future of daily urban commuting could be small, lightweight Electric L-category Vehicles (ELVs). A cost effective, energy efficient and comfortable alternative to traditional cars in cities, is at the heart of the €6.92m RESOLVE project, which included WMG at the University of Warwick.
The European project – named ‘Range of Electric Solutions for L-category Vehicles’ – designed and developed two stylish tilting four-wheeler prototype ELVs with leading European manufacturers Piaggio and KTM. These demonstrators were unveiled, and presented to representatives from the European Commission, at an event in Brussels in April 2018.
WMG was one of fourteen partners in the project, which included leading names from industry and research such as Piaggio, KTM, Bosch, Ricardo, the Austrian Institute of Technology, and the University of Florence.
New sensor tech for commercial Lithium-ion batteries finds they can be charged 5 times faster
 Researchers at WMG at the University of Warwick have developed a new direct, precise test of Lithium-ion batteries’ internal temperatures and their electrodes potentials and found that the batteries can be safely charged up to five times faster than the current recommended charging limits. The new technology works in-situ during a battery’s normal operation without impeding its performance and it has been tested on standard commercially available batteries. Such new technology will enable advances in battery materials science, flexible battery charging rates, thermal and electrical engineering of new battery materials/technology and it has the potential to help the design of energy storage systems for high performance applications such as motor racing and grid balancing.
Researchers at WMG at the University of Warwick have developed a new direct, precise test of Lithium-ion batteries’ internal temperatures and their electrodes potentials and found that the batteries can be safely charged up to five times faster than the current recommended charging limits. The new technology works in-situ during a battery’s normal operation without impeding its performance and it has been tested on standard commercially available batteries. Such new technology will enable advances in battery materials science, flexible battery charging rates, thermal and electrical engineering of new battery materials/technology and it has the potential to help the design of energy storage systems for high performance applications such as motor racing and grid balancing.
If a battery becomes over heated it risks severe damage particularly to its electrolyte and can even lead to dangerous situations where the electrolyte breaks down to form gases than are both flammable and cause significant pressure build up. Overcharging of the anode can lead to so much Lithium electroplating that it forms metallic dendrites and eventually pierce the separator causing an internal short circuit with the cathode and subsequent catastrophic failure.
Adding graphene girders to silicon electrodes could double the life of lithium batteries
New research led by WMG, at the University of Warwick has found an effective approach to replacing graphite in the anodes of lithium-ion batteries using silicon, by reinforcing the anode’s structure with graphene girders. This could more than double the life of rechargeable lithium-ion based batteries by greatly extending the operating lifetime of the electrode, and also increase the capacity delivered by those batteries.
Revolutionary method reveals impact of short circuits on battery safety
 How lithium-ion (Li-ion) batteries behave under short-circuit conditions can now be examined, using a new approach to help improve reliability and safety - developed by an international research team, including WMG at the University of Warwick.
How lithium-ion (Li-ion) batteries behave under short-circuit conditions can now be examined, using a new approach to help improve reliability and safety - developed by an international research team, including WMG at the University of Warwick.
The use of high energy density Li-ion batteries is ubiquitous – from powering portable electronics to providing grid-scale storage – but defects can lead to overheating and explosions.
Although catastrophic failure is extremely rare, recent high-profile cases including the recall of Samsung’s Galaxy Note 7 smartphone line and the grounding of an aircraft fleet highlight why it’s important to understand battery failure.
Romeo Malik, a researcher at WMG, explained the experiment:
“As the safety and reliability of batteries is paramount, it is important to know and understand the extreme scenarios of battery failure.
“In this work, we were able to see the initiation of thermal runway and how quickly it escalated to the neighbouring cells in seconds. Being able to observe and capture these rapid failures with high-speed X-ray imaging technique is amazing.”
WMG plays key role in £20 million announcement backing British Automotive battery manufacturing
 WMG at the University of Warwick are delighted to be part of a £19.4m project to support the development of next-generation electric vehicle batteries in the UK, funded through the Advanced Propulsion Centre (APC UK Ltd).
WMG at the University of Warwick are delighted to be part of a £19.4m project to support the development of next-generation electric vehicle batteries in the UK, funded through the Advanced Propulsion Centre (APC UK Ltd).
The funding will support leading edge manufacturing research focussed around Nissan's Sunderland battery manufacturing plant - the largest full scale automotive Li-ion battery manufacturing facility in Europe. WMG researchers will play a key role in helping Nissan take forward this opportunity and will receive £1m for the research.
The consortium led by Nissan with WMG at the University of Warwick, Hyperdrive, Newcastle University, and Zero Carbon Futures (ZCF), will bring together engineers, researchers, new technology and existing facilities, assets and knowledge to create and prove new and improved manufacturing processes for the next generation of automotive batteries.
WMG has particular skills around battery chemistry and the manufacturing processes used to scale this up to high volume production. WMG role in the project will be to investigate potential improvements to battery chemistry and increasing manufacturing yield, and to optimise automated manufacturing processes to enable Nissan to remain at the forefront of electric vehicle technology.
WMG hiring 120 new positions over next 100 days
 The continued success and growth of WMG, at the University of Warwick, now means that it is seeking to hire 120 new recruits over the next 100 days.
The continued success and growth of WMG, at the University of Warwick, now means that it is seeking to hire 120 new recruits over the next 100 days.
WMG is a leading centre for world class education and applied research in many sectors including: automotive, aerospace and defence, business, construction, energy and utilities, IT, security and rail. They have recently won funding for several major research projects and partnerships that builds on our large array of collaborations with new and established partners.
WMG’s Chairman Professor Lord Kumar Bhattacharyya said:
As we continue to grow and expand we are seeking individuals who thrive on a challenge and who aren’t afraid to defy conventional thinking. We are investing in, and creating, several new world-leading facilities to support these new projects. So we also need to invest in more talented people to take these projects forward and we will intend to recruit 120 of those people in the next 100 days.”
Some of the most recent developments, at WMG include a new £13.5 million Energy Innovation Centre and the £150 million National Automotive Innovation Centre.
Call for Papers - WMG Doctoral Research and Innovation Conference
 The 2nd annual WMG Doctoral Research and Innovation Conference, entitled ‘Innovation through Collaboration’, is an excellent opportunity to showcase research from both academia and industry across themes in design, materials, manufacturing, systems and business transformation.
The 2nd annual WMG Doctoral Research and Innovation Conference, entitled ‘Innovation through Collaboration’, is an excellent opportunity to showcase research from both academia and industry across themes in design, materials, manufacturing, systems and business transformation.
Organised by doctoral students, the conference will be held in the International Digital Laboratory on 30th June - 1st July, with an evening social event on the 30th.
Papers and poster presentations will take place across a wide variety of topics and awards will be presented in each theme.
Abstracts should be submitted online by 31st March.
