WMG Insights
Battery Forensics: Controlling Thermal Runaway
Justin Holloway, Professor Louis Piper and Dr Mel Loveridge
How do you make safer lithium-ion batteries?
Since their launch in 1991, the energy capacity of Lithium-ion batteries (LIBs) has substantially increased. LIBs are now used in a wide variety of portable electronic devices (such as mobile phones, watches and laptops) as well as in electric vehicles (EVs).
Although major failures of LIBs are statistically rare, they can be high-profile, with safety concerns attracting a lot of negative publicity. The Samsung Galaxy Note 7 fires led to a total product recall costing the company an estimated $17 billion. In this context, battery forensic science has emerged as a vital enabler to unearth the root causes of such failures. It is only by understanding the mechanisms behind these failures that better manufacture and safer operation of LIBs can be enabled.
Understanding and investigating thermal runaway
Thermal runaway (TR) is specifically brought about by a cascade of exothermic chain reactions, which dramatically increase the battery temperature, leading to uncontrollable temperature increases and eventual combustion.
The Forensic Analysis of Batteries (FAB) team at Warwick Manufacturing Group (WMG) is enhancing understanding of how LIBs degrade, and what the root causes of such failures are. Through use of sophisticated characterization techniques and expertise in this field, WMG can help manufacturers and users understand why and how their batteries deteriorate.

Image of (a) failed module and (b) Set-up for forensic simulation
A recent study by WMG researchers investigated a common type of battery cell known as an 18650 – these are typically used for power tools, medical devices, laptops and e-bikes. The study highlighted the importance of state-of-charge (SOC), chemistry and temperature, in driving the thermal failure mode of nickel-rich LIB cells.
The WMG methodology used was chosen to mimic the conditions of a real event, whereby cylindrical cells were exposed to continual localized heat and charging which ultimately induced catastrophic failure. By creating forensic reconstructions, researchers were able to demonstrate how to avoid thermal runaway propagation once triggered. It was associated with being able to control the exothermic reaction pathways that occur during the initial stages of thermal runaway.
The results of this study can inform effective quenching methods, which can be used to halt key stages of the thermal runaway cascade and prevent catastrophic failure of batteries.
a
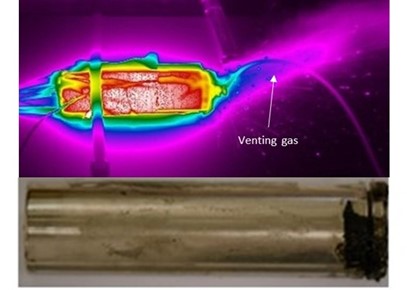
b
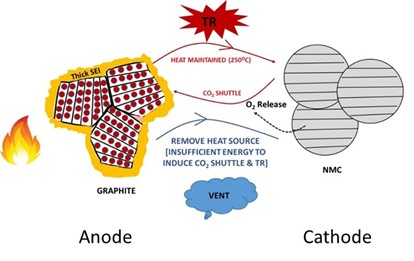
a.Thermographic image and resulting cell following thermal runaway; b.Graphical abstract of thermal runaway mitigation in a simplified cell schematic
Enabling safe and power dense batteries
The prevention of thermal runaway has become more vital than ever, as the technology is now being pushed to the limits of power and energy density for some chemistries. Examples of these include the BYD Blade cell which > 90 cm in length with a capacity of 202 Ah, which achieves greater cell density by using a cell to pack formats for EVs with no requirement for cells arranged into modules. As such, the mitigation of thermal runaway (or circumnavigating it entirely) has become paramount to ensure better safety. Establishing a better understanding of thermal runway propagation through forensic study, will enable more effective methods to be designed to deal with this potential scenario. Halting key stages of the thermal runway cascade is key to preventing catastrophic failure of batteries.
Electrode chemistries will continue to evolve and become more energy dense. Only recently have researchers become aware of the complex co-dependent behaviour between anodes and cathodes as they age.
Deeper understanding of LIBs through forensics allows a means of improving battery safety and informing design improvements for thermal management systems. Implementing learning results in the design and manufacture of better, safer and more reliable Li-ion batteries, which is of benefit to us all.
Further Information:
For more information on WMG’s forensic capabilities, visit here.
If you would like to find out about opportunities to work with WMG, please contact: wmgbusiness@warwick.ac.uk
Acknowledgements: The authors would like to thank High Value Manufacturing Catapult and The Faraday Institution SafeBatt FIRG028 project for supporting this research.
