Using adrenaline in cardiac arrests results in less than 1% more people leaving hospital alive - but nearly doubles the survivors’ risk of severe brain damage
A clinical trial of the use of adrenaline in cardiac arrests has found that its use results in less than 1% more people leaving hospital alive - but almost doubles the risk of severe brain damage for survivors of cardiac arrest. The research raises important questions about the future use of adrenaline in such cases and will necessitate debate amongst healthcare professionals, patients and the public.
Each year 30,000 people sustain a cardiac arrest in the UK and less than one in ten survive. The best chance of survival comes if the cardiac arrest is recognised quickly, someone starts cardiopulmonary resuscitation (CPR) and defibrillation (electric shock treatment) is applied without delay.
The application of adrenaline is one of the last things tried in attempts to treat cardiac arrest. It increases blood flow to the heart and increases the chance of restoring a heartbeat. However it also reduces blood flow in very small blood vessels in the brain, which may worsen brain damage. Observational studies, involving over 500,000 patients, have reported worse long-term survival and more brain damage among survivors who were treated with adrenaline.
Despite these issues, until now, there have been no definitive studies of the effectiveness of adrenaline as a treatment for cardiac arrest. This led the International Liaison Committee on Resuscitation to call for a placebo-controlled trial to establish if adrenaline was beneficial or harmful in the treatment of cardiac arrest. This “Pre-hospital Assessment of the Role of Adrenaline: Measuring the Effectiveness of Drug administration In Cardiac arrest (PARAMEDIC2)” trial was undertaken to determine if adrenaline is beneficial or harmful as a treatment for out of hospital cardiac arrest.
The trial was funded by the National Institute for Health Research, sponsored by the University of Warwick and led by researchers in the University’s Clinical Trials Units – part of Warwick Medical School. The trial ran from December 2014 through October 2017. It was conducted in 5 National Health Service Ambulance Trusts in the United Kingdom, and included 8000 patients who were in cardiac arrest. Patients were allocated randomly to be given either adrenaline or a salt-water placebo and all those involved in the trial including the ambulance crews and paramedics were unaware which of these two treatments the patient received.
The results of the trial have now been published in the New England Journal of Medicine (NEJM) on Thursday 19th July 2018 in an article entitled “A Randomized Trial of Epinephrine in Out-of-Hospital Cardiac Arrest”
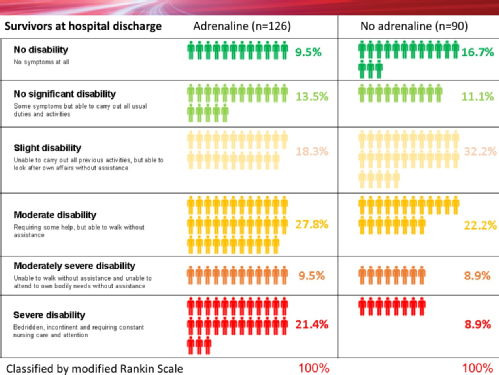
Of 4012 patients given adrenaline, 130 (3.2%) were alive at 30 days compared with 94 (2.4%) of the 3995 patients who were given placebo. However, of the 128 patients who had been given adrenaline and who survived to hospital discharge 39 (30.1%) had severe brain damage, compared with 16 (18.7%) among the 91 survivors who had been given a placebo. In this study a poor neurological outcome (severe brain damage) was defined as someone who was in a vegetative state requiring constant nursing care and attention, or unable to walk and look after their own bodily needs without assistance.
The reasons why more patients survived with adrenaline and yet had an increased chance of severe brain damage are not completely understood. One explanation is that although adrenaline increases blood flow in large blood vessels, it paradoxically impairs blood flow in very small blood vessels, and may worsen brain injury after the heart has been restarted. An alternative explanation is that the brain is more sensitive than the heart to periods without blood and oxygen and although the heart can recover from such an insult, the brain is irreversibly damaged.
Professor Gavin Perkins Professor of Critical Care Medicine in Warwick Medical School at the University of Warwick (and the lead author on the paper) said:
“We have found that the benefits of adrenaline are small – one extra survivor for every 125 patients treated – but the use of adrenaline almost doubles the risk of a severe brain damage amongst survivors.”
“Patients may be less willing to accept burdensome treatments if the chances of recovery are small or the risk of survival with severe brain damage is high. Our own work with patients and the public before starting the trial identified survival without brain damage is more important to patients than survival alone. The findings of this trial will require careful consideration by the wider community and those responsible for clinical practice guidelines for cardiac arrest.”
Professor Jerry Nolan, from the Royal United Hospital Bath (and a co-author on the paper) said:
"This trial has answered one of the longest standing questions in resuscitation medicine. Taking the results in context of other studies, it highlights the critical importance of the community response to cardiac arrest. Unlike adrenaline, members of the public can make a much bigger difference to survival through learning how to recognise cardiac arrest, perform CPR and deliver an electric shock with a defibrillator."
Notes for editors
1) Cardiac arrest means that the heart has stopped pumping blood around the body. Although ‘heart attack’ is often used to refer to a sudden cardiac arrest, this is incorrect. A heart attack (or myocardial infarction to use the medical term) occurs when an artery supplying the heart with blood becomes blocked. This usually causes chest pain and leads to damage to some of the muscle of the heart. It may cause cardiac arrest, particularly in the early stages, but this is not inevitable.
https://www.resus.org.uk/faqs/faqs-cpr/
https://www.bhf.org.uk/heart-matters-magazine/medical/heart-attack-and-cardiac-arrest
2) Epinephrine is the US drug name for adrenaline
3) The most effective treatments are recognising cardiac arrest and dialling 999 (1 extra survivor for every 11 people treated), starting CPR (1 extra survivor for every 15 people treated), public access defibrillation (1 extra survivor for every 5 people treated). Current guidelines advise that adrenaline is given if these initial treatments are unsuccessful : https://www.resus.org.uk/resuscitation-guidelines/adult-advanced-life-support
4) The study was designed and run in accordance with the EU Clinical Trials Directive, UK Clinical Trials Regulations and principles of Good Clinical Practice. It was Funded by the National Institute for Health Research HTA Programme (12/127), ISRCTN73485024 and was sponsored by the University of Warwick. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
5)The participating ambulance services were: London Ambulance Service, North East Ambulance Service, South Central Ambulance Service, the Welsh Ambulance Service, and the West Midlands Ambulance Service
6)The authors of the PARAMEDIC 2 paper were: Gavin Perkins, Chen Ji, Charles Deakin, Tom Quinn, Jerry P Nolan, Charlotte Scomparin, Scott Regan, John Long, Anne Slowther, Helen Pocock, John JM Black, Fionna Moore, Rachael T Fothergill, Nigel Rees, Lyndsey O’Shea, Mark Docherty, Imogen Gunson, Kyee Han, Karl Charlton, Judith Finn, Stavros Petrou, Nigel Stallard, Simon Gates, Ranjit Lall.
7)The PARAMEDIC2 research team were supported by: NIHR Comprehensive Research Network, Health Care and Research Wales, University of Warwick, Kingston University and St George’s, University of London, NIHR Southampton Respiratory Biomedical Research Unit, University of Bristol, University Hospitals Birmingham, Royal United Hospital, Bath, South East Coast Ambulance Service, Curtin University, Perth, Australia, Monash University, Melbourne, Australia, The Intensive Care Foundation
8) The reach funder was the National Institute for Health Research (NIHR): improving the health and wealth of the nation through research. Established by the Department of Health and Social Care, the NIHR:
· funds high quality research to improve health
· trains and supports health researchers
· provides world-class research facilities
· works with the life sciences industry and charities to benefit all
· involves patients and the public at every step
For further information, visit the NIHR website www.nihr.ac.uk
PR PJD 17th July 2018
For further information please contact:
Peter Dunn, Director of Press and Media Relations
University of Warwick
Tel UK 024 76523708 office 07767 655860 mobile
Tel Overseas: +44 (0)24 76523708 office +44 (0)7767 655860 mobile/cell
Email p dot j dot dunn at warwick dot ac dot uk