News
Inverted membranes by ion soft landing
Costantini and co-workers report in Advanced Materials on the fabrication of inverted crystalline membranes of sodium dodecyl sulfate by ESI deposition.
Smart Materials with Triggerable Membrane Interactions
The Gibson group (in collaboration with Alison Rodger) investigate how stimuli responsive polymers can be used to modulate lipophilicity.
Structural studies on a Meningitis B vaccine
Results by MOAC and chemistry PhD student Angela Martino and her supervisor Alison Rodger were published this week on 4CMenB (a new meningococcal B vaccine).
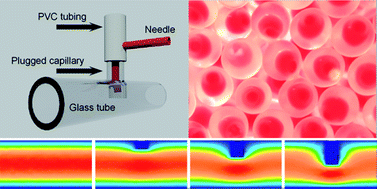
The Bonlab goes DIY with a microfluidic device for fabrication of double emulsion droplets and polymer microcapsules
The Bonlab lead by assoc. prof. Stefan Bon publishes in Polymer Chemistry how to make a DIY microfluidic device to fabricate droplets-in-droplets. They demonstrate that by using a syringe needle, plastic tubing, two glass capillaries and epoxy glue a microfluidic device can be fabricated straightforwardly that allows for the production of double emulsions, or in other words the generation of droplets-in-droplets. The device in essence is a serial combination of droplet generation by co-flow and a T-junction. To reduce potential issues with channel wetting, we established that an “obstructed” T-junction outperformed a conventional T-junction. They illustrate the versatility of our device through production of a range of polymer microcapsules, including ones that contain a waterborne dispersion of colour changing pigment, and microcapsules with compartmentalized ferrofluidic segments, that is capsules that contain more than one droplet of ferrofluid.
To read the paper: http://dx.doi.org/10.1039/C2PY00605G
To find out more about the BonLab: http://www.bonlab.info, Twitter: Bonlab, Youtube: BonlabTV
Resolutely Pure Helices
Warwick antibiotic complexes appear in Nature Chemistry News & Views article by Janice Aldrich-Wright.
- "...a simplicity and an elegance of design that should be a source of inspiration for future studies...
- ...easily tuned to explore structure–function relationships that are crucial in biological applications...
- ...good antibiotic activity against...MRSA and E. coli as well as low toxicity to Caenorhabditis elegans.
- ...potential to develop into a family of cost-effective antibiotics."
Stimuli Responsive Polymers Tuned for Specific Intracellular Degradation
The Gibson group have reported in Chemical Commmunications on a new route to obtain polymers containing disulfide linkages in their backbone. These linkages are appealing for drug delivery applications as they are stable in the blood stream but can be specifically degraded inside cells. Traditional controlled radical polymerizations produce all-carbon backbones which do not degrade but, in this paper, the authors demonstrate how a 2-stage polymerization process can be used to incorporate disulfides. Furthmore this allows the use of functional monomers which result in 'smart' materials capable of responding to thermal gradients.
This was published in Chemical Communications link
More information on the Gibson Group can be found here link
Quadruple hydrogen bond polymer colloids HIPE up gels
Scientists in the team of assoc. prof. Stefan Bon (BonLab) have developed a convenient route to organogels templated by high internal phase emulsions (HIPEs) of water droplets which they refer to as HIPE- gels. Key is the use of a waterborne polymer latex loaded with a multiple hydrogen bond (MHB) array functionality - here 2-ureido-4[1H] pyrimidinone (UPy). Upon contact with an organic phase is a good solvent for the polymer (by shaking), swelling, and subsequent partial disentanglement of polymer chains originating from and wrapped up in the form of a latex particle, and phase transfer of polymer into the organic phase occurs. This process promotes the formation of a cross-linked swollen network in the form of an organogel through quadruple hydrogen bond interactions. Typically a HIPE-gel formulation contains 85vol% dispersed water droplets in 15vol% toluene at an original loading of 0.1wt% of UPy-functionalized latex particles in the water phase. They demonstrate that the HIPE-gels can be used as a soft matter storage and slow release material. The closed-cell compartmentalized water droplets remain intact upon submersion of the HIPE-gel into water, even under high osmotic pressure gradients. Upon drying the HIPE-gel a self-supporting porous monolith is obtained. They believe that the approach to the fabrication of the HIPE gels opens routes for potential applications in medical injectable gels, pressure sensors, and other interesting multiphase soft matter materials.
The work is published in chemical communications, link
More on the BonLab, link
‘Left-handed iron corkscrews’ point the way to new weapon in battle against superbugs like MRSA

 Scientists at the University of Warwick have taken inspiration from corkscrew structures found in nature to develop a new weapon in the fight against infections like E-coli and MRSA.
Scientists at the University of Warwick have taken inspiration from corkscrew structures found in nature to develop a new weapon in the fight against infections like E-coli and MRSA.
Researchers have created a new synthetic class of helix-shaped molecules which they believe could be a key tool in the worldwide battle against antibiotic resistance. By twisting molecules around iron atoms they have created what they term ‘flexicates’ which are active against MRSA and E-coli - but which also appear to have low toxicity , reducing the potential for side effects if used in treatment. The work is published in Nature Chemistry.
The new structures harness the phenomenon of ‘chirality’ or ‘handedness’ whereby the corkscrew molecules could be left-handed or right-handed. By making the most effective ‘hand’ to attack a specific disease, the University of Warwick research paves the way towards a more targeted approach to killing pathogens. In the case of E-coli and MRSA, it is the left ‘hand’ which is most effective.
Professor Peter Scott of the University of Warwick’s chemistry department said although this particular study concentrated on flexicates’ activity against MRSA and E-coli, the new method of assembly could also result in new treatments for other diseases.
“It’s a whole new area of chemistry that really opens up the landscape to other practical uses. These new molecules are synthetically flexible, which means that with a bit of tweaking they can be put to use against a whole host of different diseases, not just bugs like MRSA which are rapidly developing resistance to traditional antibiotics. Flexicates are also easier to make and produce less waste than many current antibiotics.”
Scientists have long been able to copy nature’s corkscrew-shaped molecules in man-made structures known as helicates – but they have thus far not been able to use them in fighting diseases. One of the key issues is the problem of handedness. Sometimes ‘left-handed’ molecules in drugs are the most effective at combating some disease, while sometimes the ‘right-handed’ version works best. Until now, scientists working with helicates have found it difficult to make samples containing just one type of corkscrew; either the right- or left-handed twist.
With flexicates, the University of Warwick scientists have succeeded in making samples containing just one type of twist – resulting in a more targeted approach which would allow the drug dosage to be halved. Flexicates solve other problems encountered by helicates, as they are easier to optimise for specific purposes, are better absorbed by the body and are easier to mass-produce synthetically.
Professor Scott said: “Drugs often have this property of handedness - their molecules can exist in both right and left handed versions but the body prefers to use only one of them. For this reason, drug companies have to go to the trouble of making many traditional molecules as one hand only. What we have done is solve the ‘handedness’ problem for this new type of drug molecule. By getting the correct hand we can halve the drug dose, which has the benefits of minimising side effects and reducing waste. For patients, it’s safer to swallow half the amount of a drug. Our work means that we can now make whichever hand of the corkscrew we want, depending on the job we require it to do.”
Notes to editors
The study, entitled Optically pure, water-stable metallo-helical ‘flexicate’ assemblies with antibiotic activity, is published in Nature Chemistry.
The research was also supported financially by EPSRC.
It is authored by Peter Scott, Suzanne Howson, Guy Clarkson and Alison Rodger from the University of Warwick, Albert Bolhuis from the University of Bath and Viktor Brabec and Jaroslav Malina from the Academy of Sciences of the Czech Republic.
When the paper is published it can be retrieved at http://dx.doi.org/10.1038/NCHEM.1206
Contact details
Professor Peter Scott is available on +44 (0) 24 7652 3238 or peter.scott@warwick.ac.uk
University of Warwick press officer Anna Blackaby is available on + 44 (0) 2476 575910 or + 44 (0)7785 433155 or a.blackaby@warwick.ac.uk
Front Page Artwork by Elisabeth Heissler http://ehgraphicdesign.co.uk/
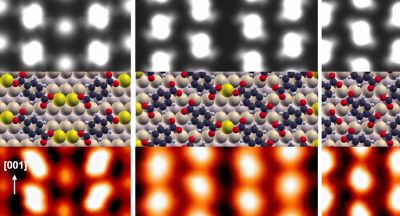
At surfaces it's different
Costantini and collaborators have reported in a special themed issue of Chemical Communications about a novel chemical pathway observed only in the presence of a metal substrate. In solution chemistry, assuming no kinetic limitations, the thermodynamic product is formed independently of the absolute reactant concentration. However, inclusion of a metallic substrate introduces a further variable which ultimately defines the chemistry observed. In their recent work, terephthalic acid was deposited onto a Cu(110) substrate, where, at low surface coverages, 2-dimensional metal-organic structures form. However, with increasing coverage, the interaction between molecule and metal induces the formation of a denser, less energetically-favoured hydrogen-bonded network.
The article can be read here .
.
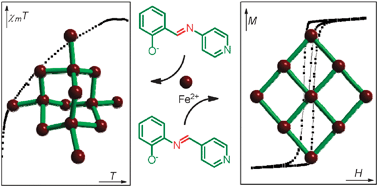
Simple compounds, hard magnets
New magnetic materials have been created from simple components by Lihong Li, a Warwick Postgraduate Research Fellow and co-workers in the Scott group at Warwick University. The resarchers have engineered magnetic diamond-like 3D networks and layered 2D net structures. A rare example of a molecular hard magnet (which like a regular magnet keeps its magnetic properties over time) is produced. The chemistry is simple, reliable and scalable so the group hope to make many new materials in the future for applications in data storage, and quantum computing. The work is published in Chemical Communications.
Size is Key Parameter in 'Smart Nanoparticles'
Gibson  and O'Reilly groups have published a manuscript in Chemical Communications which demonstrate the critical importance of nanoparticle diameter on its thermo-responsive behaviour, as demonstrated using a panel of gold and self-assembled polymer nanoparticles. These findings have implications for the rationale design of 'smart' nanomaterials for biotechnological applications.
and O'Reilly groups have published a manuscript in Chemical Communications which demonstrate the critical importance of nanoparticle diameter on its thermo-responsive behaviour, as demonstrated using a panel of gold and self-assembled polymer nanoparticles. These findings have implications for the rationale design of 'smart' nanomaterials for biotechnological applications.
Fabrication of clay armored "soft" polymer latexes through Pickering emulsion polymerization
The BonLab reports on the fabrication of “soft” nanocomposite clay armored polymer latexes with their latest work published in the ACS journal Macromolecules.
Laponite clay XLS is used as stabilizer in the Pickering emulsion polymerization of a variety of monomer mixtures, that is, methyl methacrylate and n-butyl acrylate, styrene and n-butyl acrylate, and styrene and 2-ethylhexyl acrylate. Overall solids contents of the hybrid latexes in complete absence of coagulation of up to 24 wt % are reported under batch conditions. Key mechanistic aspects of the Pickering emulsion polymerization process are discussed. The use of monomers that have high water solubility and are prone to hydrolyze under basic conditions, for example methyl methacrylate, should be restricted. The use of small amounts of methacrylic acid as auxiliary monomer promotes clay adhesion to the surface of the particles in the Pickering emulsion (co)polymerization of hydrophobic monomers. Detailed kinetic studies at both 60 and 80 °C of the Pickering emulsion copolymerization of styrene and n-butyl acrylate (Sty:BA = 0.67 w/w) are reported, with varying amounts of Pickering stabilizer. The Laponite clay discs play a crucial role in the particle formation (nucleation) stage of the Pickering emulsion polymerization process. Use of increasing amounts leads to smaller average particle sizes but inflicts longer nucleation periods, thereby broadening the particle size distributions. We report the occurrence of a catastrophic coagulation phenomenon for Pickering emulsion polymerizations carried out at a low initiator (ammonium persulfate) flux at 60 °C, for a small window of concentrations of Laponite clay discs.
Read the paper: http://dx.doi.org/10.1021/ma201691u
More info on the BonLab: http://www.bonlab.info