Current Research
Why FtsZ
FtsZ is now one of the most studied bacterial proteins for a number of reasons:
1) FtsZ make up the main cytoskeletal element Z-ring in bacteria, which palys an important role in the control of cell division.
2) FtsZ is a highly conserved protein that is found in most of the major groups of bacteria.
3) FtsZ is an ancestral homologue of tubulin, even could be the evolutionary precursor to tubulin and microtubules.
4) FtsZ could be the target for the development of new antibiotics.
FtsZ in bacterial cell division
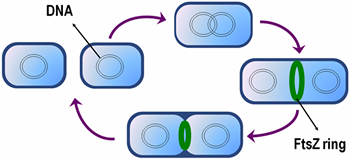
Like tubulin, FtsZ is a GTPase, which is capable of GTP-induced self-assembly into higher-order polymers. The earliest event in bacterial cytokinesis is the localization of FtsZ to the future division site, where it assembles into a ring-like structure called the Z-ring. The Z-ring then reduces in diameter during cell division, drawing the cytoplasmic membrane inwards.
A typical cell cycle in E. coli
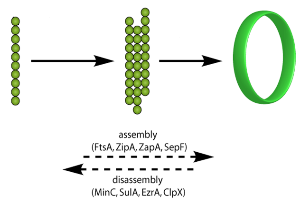
In order to enable cytokinesis to be completed, the Z-ring also need to recruit other accessory proteins to assemble the cell division machinery, by either direct or indirect interaction with FtsZ. These proteins can be conveniently grouped into inhibitors and supporters of FtsZ polymerization.
FtsZ assembly and disassembly
Focus in my work
It seems increasingly likely that the formation of bundles through lateral interactions between FtsZ polymers is critical to the function of Z-ring, so I am interested in studying promoters of FtsZ polymerization. The current work involves studying how YgfE, the putative ZapA orthologue in E. coli, promote FtsZ association. Particularly, I use linear dichroism, as a novel technique, to monitor the changes in the FtsZ polymers.
References:
1. Adams, D.W. & Errington, J. Bacterial cell division: assembly, maintenance and disassembly of the Z ring. Nature Reviews Microbiology (2009) 7: 642-653.
2. Margolin, W. FtsZ and the division of prokaryotic cells and organelles. Nature Reviews Molecular Cell Biology (2005) 6: 826-871.
3. Small, E. et al. FtsZ polymer-bundling by the Escherichia coli ZapA orthologue, YgfE, involves a conformational change in bound GTP. J. Mol. Biol. (2007) 369: 210-221.
4. Gueiros-Filho, F.J. & Losick, R. A widely conserved bacterial cell division protein that promotes assembly of the tubulin-like protein FtsZ. Genes & Dev. (2002) 16: 2544-2556.