Yuko P. Y. Lam

Research Fellow
FT-ICR Mass Spectrometry Group
Current Research Projects:
Developing two dimensional mass spectrometry (2DMS): the next dimension of proteomics
Mass spectrometry has been widely used to observe the interactions between compounds and proteins. For complex samples, MS co-operates with liquid chromatography (LC) to separate proteins and peptides in samples prior to MS analysis. An LC system is an essential tool for MS in complex sample analysis, the solvents applied in the system, however, are often not ideal for all analytes of interest, and frequently disrupt important biological processes such as co-factor or therapeutic target binding. A new analytical technique – two dimensional mass spectrometry (2DMS) provides an extra dimension of separation in MS, allows the study of the wide range of analytes observed in complex proteomic systems, from extreme’s in hydrophobicity/philicity, to interactions between exogenous compounds and biomolecules.
2DMS spectrum:

Exploring the aggregation/inhibition pathways of amyloid protein and the effects of deamidation on amyloid protein through FTICR-MS
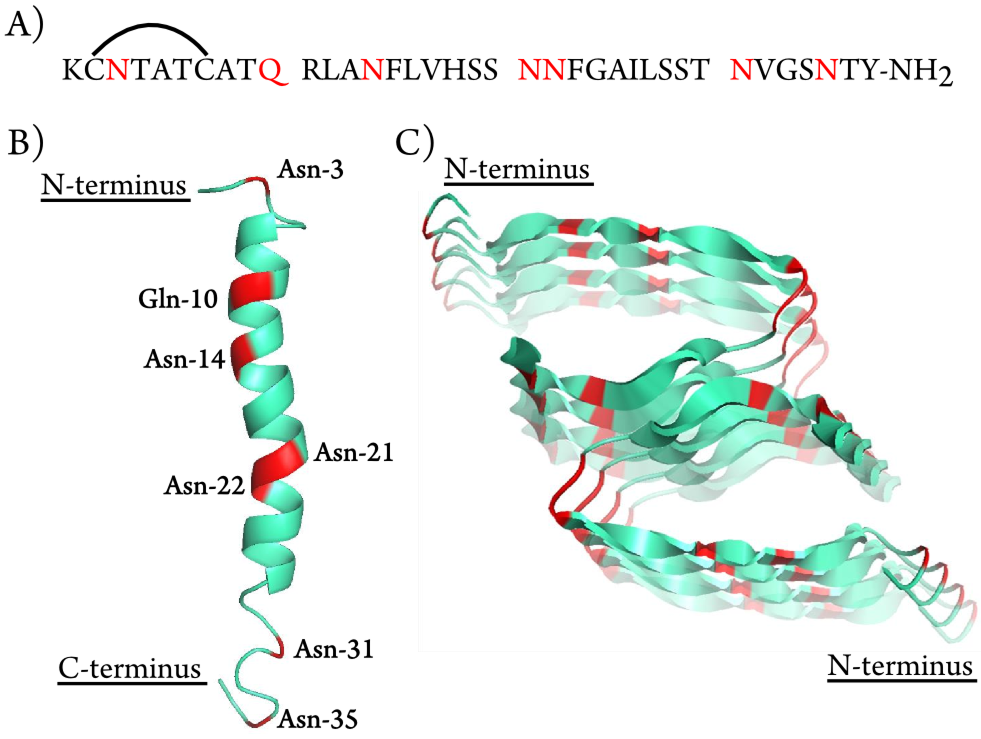
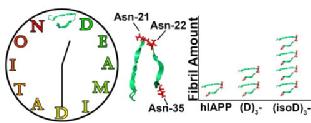
Amyloid proteins aggregation is a hallmark in lots of diseases, i.e. Alzheimer's Disease, Parkinson Disease, and type II diabetes. The aggregation mechanisms of amyloid proteins have been studied and discussed for a long period of time, however, most of them have not been conclusively determined yet. With a better understanding of the aggregation mechanisms helps the development of prevention and treatment of amyloid diseases. Thus, we apply the ultra-high resolution Fourier Transform Ion Cyclotron Resonance Mass Spectrometry (FTICR MS) for the analysis of amyloid protein aggregation.
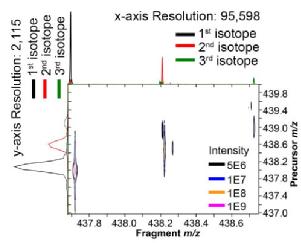
Deamidation is one of the protein ageing modifications, which has been shown to have a strong correlation with amyloid diseases. Determining precise deamidation site is critical for drug development but difficult in lots of analytical instruments. For MS analysis, deamidation causes the monoisotopic peak shift of +0.984 Da which is very close to the 2nd isotopic peak +1.003 Da. Ultra-high resoltuion FTICR MS is applied to well-resolved the 0.019 Da mass different.
Presentations
2019/09 Oral Presentation at the 40th British Mass Spectrometry Society (BMSS) Conference
2019/06 Oral Presentation at the 67th American Society for Mass Spectrometry (ASMS) Annual Conference
2019/04 Poster Presentation at the Celebration of Native Mass Spectrometry Conference
2018/10 Poster Presentation at the 17th East Midlands Proteomics Workshop (EMPW)
2018/08 Oral Presentation at the 22nd International Mass Spectrometry Conference (IMSC)
2017/11 Poster Presentation at the 16th East Midlands Proteomics Workshop (EMPW)
2017/09 Poster Presentation at the 38th British Mass Spectrometry Society (BMSS) Conference
2017/07 Flash & Poster Presentation at the Analytical Research Forum (ARF) 2017
2017/06 Oral Presentation at the 65th American Society for Mass Spectrometry (ASMS) Annual Conference
2016/11 Poster Presentation at the 15th East Midlands Proteomics Workshop (EMPW)
2016/09 Poster Presentation at the 37th British Mass Spectrometry Society (BMSS) Conference
2016/04 Poster Presentation at the 9th Isolated Biomolecules and Biomolecular Interactions (IBBI) Conference
2016/04 - Oral Presentation at the 12th European Fourier Transform Mass Spectrometry (EFTMS) Conference
2015/11 - Oral Presentation at the 14th East Midlands Proteomics Workshop (EMPW)
2015/09 - Poster Presentation at the 36th British Mass Spectrometry Society (BMSS) annual meeting


Presentation Awards
2017 - Awarded for the flash presentation runner up prize at the Analytical Reserach Forum 2017
2015 - Awarded for the best talk at the 14th East Midlands Proteomics Workshop (EMPW)
2015 - Awarded for the Bordoli prize for best young reseracher Poster Presentation at the 36th British Mass Spectrometry Society (BMSS) annual meeting
Publication
- Lam, Y.P.Y.; Chiu, C.K.C.; Wootton, C.A.; Li, M.; Hands-Portman, I.; Barrow, M.P.; O’Connor, P.B. Does Deamidation affect the Inhibitory Mechanism towards Amyloid Protein Aggregation? Chem. Commun. 2020. DOI: https://doi.org/10.1039/D0CC03548C
- Lam, Y. P. Y.; Wootton, C. A.; Hands-Portman, I.; Wei, J.; Chiu, C. K. C.; Romero-Canelon, I.; Lermyte, F.; Barrow, M. P.; O’Connor, P. B., Determination of the Aggregate Binding Site of Amyloid Protofibrils Using Electron Capture Dissociation Tandem Mass Spectrometry. J. Am. Soc. Mass. Spectrom. 2020, 31 (2), 267-276. DOI: http://doi.org/10.1021/jasms.9b00053
- Chiu, C. K. C.; Lam, Y. P. Y.; Wootton, C. A.; Barrow, M. P.; Sadler, P. J.; O’Connor, P. B., Metallocomplex–Peptide Interactions Studied by Ultrahigh Resolution Mass Spectrometry. J. Am. Soc. Mass. Spectrom. 2020, 31 (3), 594-601. DOI: http://doi.org/10.1021/jasms.9b00054
- van Agthoven, M. A.; Lam, Y. P. Y.; O’Connor, P. B.; Rolando, C.; Delsuc, M.-A., Two-dimensional mass spectrometry: new perspectives for tandem mass spectrometry. Eur. Biophys. J. 2019, 48 (3), 213-229. DOI: http://doi.org/10.1007/s00249-019-01348-5
- Lermyte, F.; Everett, J.; Lam, Y. P. Y.; Wootton, C. A.; Brooks, J.; Barrow, M. P.; Telling, N. D.; Sadler, P. J.; O’Connor, P. B.; Collingwood, J. F., Metal Ion Binding to the Amyloid β Monomer Studied by Native Top-Down FTICR Mass Spectrometry. J. Am. Soc. Mass. Spectrom. 2019. DOI: http://doi.org/10.1007/s13361-019-02283-7
- Chen, P.; Zhang, D.; Li, M.; Wu, Q.; Lam, Y. P. Y.; Guo, Y.; Chen, C.; Bai, N.; Malhotra, S.; Li, W.; O'Connor, P. B.; Fu, H., Discovery of novel, potent, isosteviol-based antithrombotic agents. Eur. J. Med. Chem. 2019, 111722. DOI: http://doi.org/10.1016/j.ejmech.2019.111722
- van Agthoven, M. A.; Lynch, A. M.; Morgan, T. E.; Wootton, C. A.; Lam, Y. P. Y.; Chiron, L.; Barrow, M. P.; Delsuc, M.-A.; O’Connor, P. B., Can Two-Dimensional IR-ECD Mass Spectrometry Improve Peptide de Novo Sequencing?Anal. Chem. 2018, 90 (5), 3496-3504. DOI: http://doi.org/10.1021/acs.analchem.7b05324
- Lam, Y. P. Y.; Wootton, C. A.; Hands-Portman, I.; Wei, J.; Chiu, C. K. C.; Romero-Canelon, I.; Lermyte, F.; Barrow, M. P.; O’Connor, P. B., Does Deamidation of Islet Amyloid Polypeptide Accelerate Amyloid Fibril Formation? Chem. Commun. 2018, 54 (98), 13853-13856. DOI: http://doi.org/10.1039/C8CC06675B
- Floris, F.; van Agthoven, M. A.; Chiron, L.; Wootton, C. A.; Lam, Y. P. Y.; Barrow, M. P.; Delsuc, M.-A.; O’Connor, P. B., Bottom-Up Two-Dimensional Electron-Capture Dissociation Mass Spectrometry of Calmodulin. J. Am. Soc. Mass. Spectrom. 2018, 29 (1), 207-210. DOI: http://doi.org/10.1007/s13361-017-1812-y
- Zhang, P.; Chiu, C. K.; Huang, H.; Lam, Y. P. Y.; Habtemariam, A.; Malcomson, T.; Paterson, M. J.; Clarkson, G. J.; O'Connor, P. B.; Chao, H.; Sadler, P. J., Organoiridium photosensitizers induce specific oxidative attack on proteins within cancer cells. Angew. Chem. Int. Ed. 2017, 56 (47), 14898-14902. DOI: http://doi.org/10.1002/anie.201709082
- Wootton, C. A.; Lam, Y. P. Y.; Willetts, M.; van Agthoven, M. A.; Barrow, M. P.; Sadler, P. J.; Peter, B., Automatic assignment of metal-containing peptides in proteomic LC-MS and MS/MS data sets. Analyst 2017, 142 (11), 2029-2037. DOI: http://doi.org/10.1039/C7AN00075H
- Wei, J.; Antzutkin, O. N.; Filippov, A. V.; Iuga, D.; Lam, Y. P. Y.; Barrow, M. P.; Dupree, R.; Brown, S. P.; O’Connor, P. B., Amyloid Hydrogen Bonding Polymorphism Evaluated by 15N {17O} REAPDOR Solid-State NMR and Ultra-High Resolution Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. J. Biochem. 2016, 55 (14), 2065-2068. DOI: http://doi.org/10.1021/acs.biochem.5b01095
- Simon, H.; van Agthoven, M.; Lam, Y. P. Y.; Floris, F.; Chiron, L.; Delsuc, M.-A.; Rolando, C.; Barrow, M.; O'Connor, P., Uncoiling collagen: a multidimensional mass spectrometry study. Analyst 2016, 141 (1), 157-165. DOI: http://doi.org/10.1039/C5AN01757B
- Floris, F.; van Agthoven, M.; Chiron, L.; Soulby, A. J.; Wootton, C. A.; Lam, Y. P. Y.; Barrow, M. P.; Delsuc, M.-A.; O’Connor, P. B., 2D FT-ICR MS of calmodulin: a top-down and bottom-up approach. J. Am. Soc. Mass. Spectrom. 2016, 27 (9), 1531-1538. DOI: http://doi.org/10.1007/s13361-016-1431-z
- Perez Hurtado, P.; Lam, Y. P. Y.; Kilgour, D.; Bristow, A.; McBride, E.; O’Connor, P. B., Use of high resolution mass spectrometry for analysis of polymeric excipients in drug delivery formulations. Anal. Chem. 2012, 84 (20), 8579-8586. DOI: http://doi.org/10.1021/ac301576h
ORCHiD Link:
https://orcid.org/0000-0001-5486-091X
ORCiD QR Code

