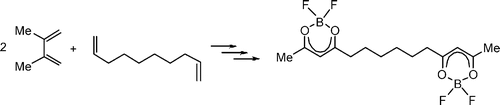Stefan Roesner
Assistant Professor
 Stefan Roesner received his Diploma in chemistry from Philipps-University Marburg, Germany in 2010. He moved to the UK and completed a Ph.D. in organic chemistry with Prof. Varinder Aggarwal at the University of Bristol in 2014. After postdoctoral studies at the Massachusetts Institute of Technology under the supervision of Prof. Stephen Buchwald, he returned in 2016 to the UK to become Senior Research Fellow in the Shipman group. In October 2021 he was promoted to become Assistant Professor.
Stefan Roesner received his Diploma in chemistry from Philipps-University Marburg, Germany in 2010. He moved to the UK and completed a Ph.D. in organic chemistry with Prof. Varinder Aggarwal at the University of Bristol in 2014. After postdoctoral studies at the Massachusetts Institute of Technology under the supervision of Prof. Stephen Buchwald, he returned in 2016 to the UK to become Senior Research Fellow in the Shipman group. In October 2021 he was promoted to become Assistant Professor.
S dot Roesner at warwick dot ac dot uk
024 765 22758
Office C309
My office hours are Mon-Fri 9 am - 5 pm
Groups at Warwick
Biography
- 2010, Dipl. Chem. from Philipps-University Marburg, Germany
- 2014, PhD from University of Bristol, UK
- 2015-2016, Postdoctoral Research Associate, Massachusetts Institute of Technology, USA
- 2016-2021, Senior Research Fellow, University of Warwick, UK
- since 2021, Assistant Professor, University of Warwick, UK
I am working on the following projects at the moment:
- Functionalisation of cyclic hydrazines
- Synthesis of carbolines and related heterocycles
I am currently interested in the following areas:
- Continuous-flow synthesis
- Metal-catalysed cross-coupling reactions
- Synthesis of functionalised heterocycles
I am module lader for CH161 Introduction to Organic Chemistry, and teach the third part of this course
Get in touch if you consider applying for PhD studies. The University of Warwick offers several schemes to fund home and international students.
Publications
• G. J. Clarkson, S. Roesner, ChemRxiv 2022, DOI: 10.26434/chemrxiv-2022-63g8k. ‘Synthesis of Benzofuropyridines and Dibenzofurans by a Metalation/Negishi Cross-Coupling/SNAr Reaction Sequence’. [Abstract]

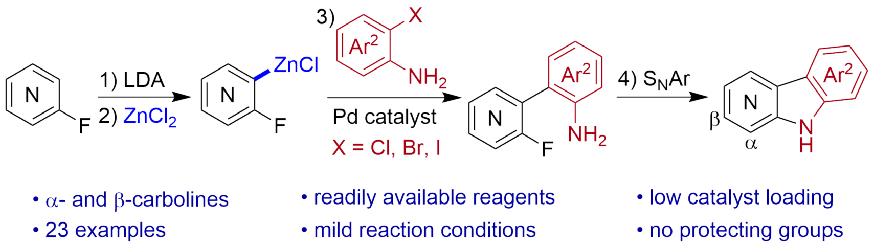
• S. Sathiyalingam, S. Roesner, Adv. Synth. Catal. 2022, 364, 1769. ‘Synthesis of α- and β-Carbolines by a Metalation/Negishi Cross-Coupling/SNAr Reaction Sequence’. [Abstract]
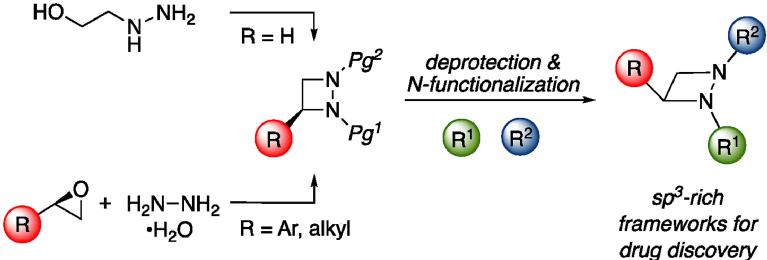
• C. Dean, S. Roesner, S. Rajkumar, G. J. Clarkson, M. Jones, M.; Shipman, Tetrahedron 2021, 79, 131836. 'Synthesis of sp3-rich Chemical Libraries based upon 1,2-Diazetidines'. [Abstract]
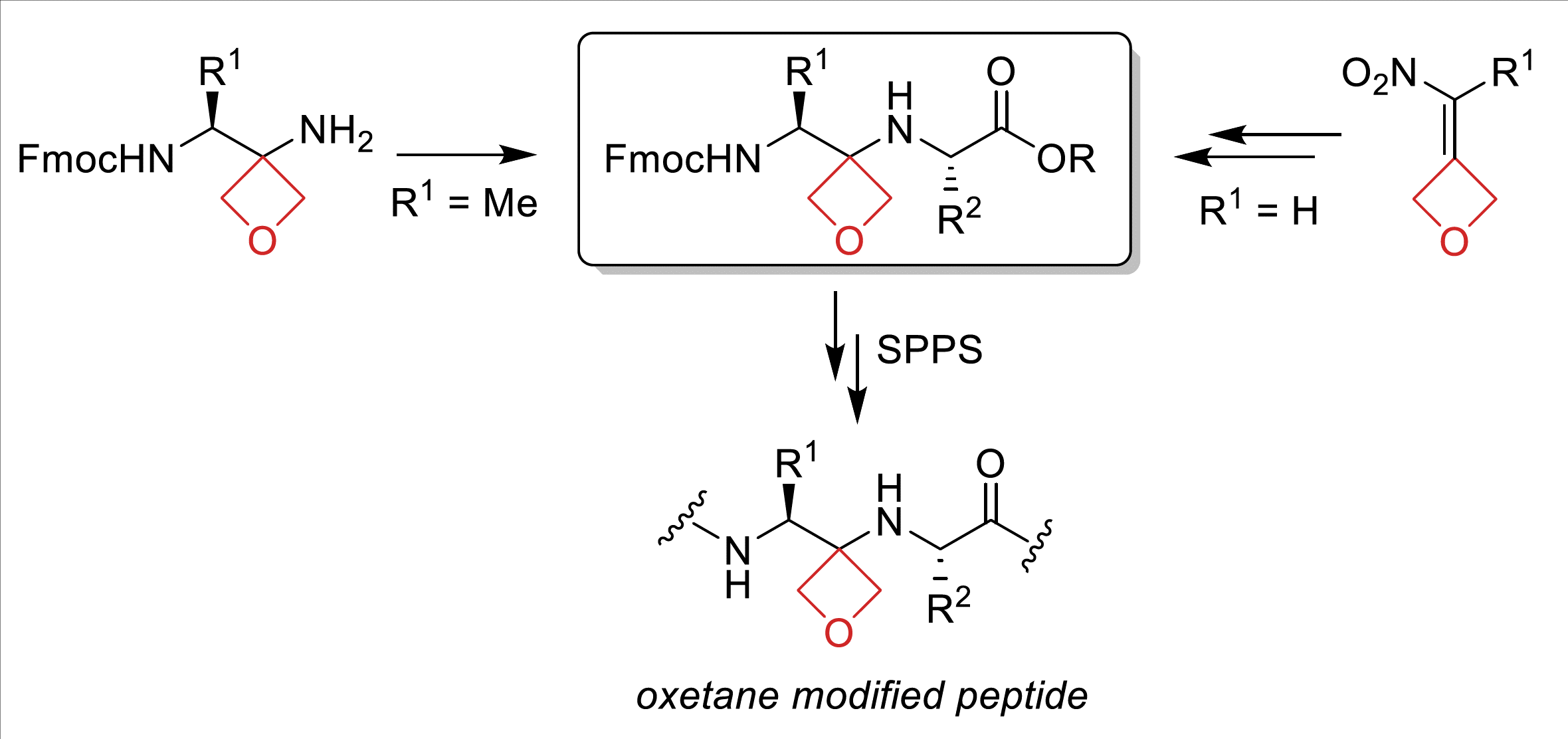
• S. Roesner, J. D. Beadle, L. K. B. Tam, I. Wilkening, G. J. Clarkson, P. Raubo, M. Shipman, Org. Biomol. Chem. 2020, 18, 5400. 'Development of Oxetane Modified Building Blocks for Peptide Synthesis'. [Abstract]
• C. Dean, R. Sundaram, S. Roesner, N. Carson, G. J. Clarkson, M. Wills, M. Jones, M. Shipman, Chem. Sci. 2020, 11, 1636. 'Readily accessible sp3-rich cyclic hydrazine frameworks exploiting nitrogen fluxionality'. [Abstract]
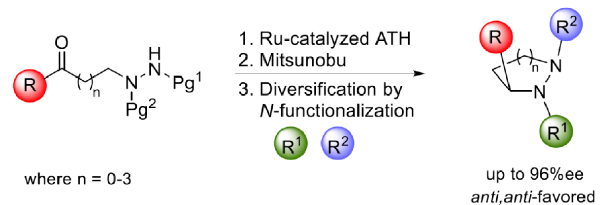
• S. Roesner, G. J. Saunders, I. Wilkening, E. Jayawant, J. V. Geden, P. Kerby, A. M. Dixon, R. Notman, M. Shipman, Chem. Sci. 2019, 10, 2465. ‘Macrocyclisation of Small Peptides Enabled by Oxetane Incorporation’. [Abstract]
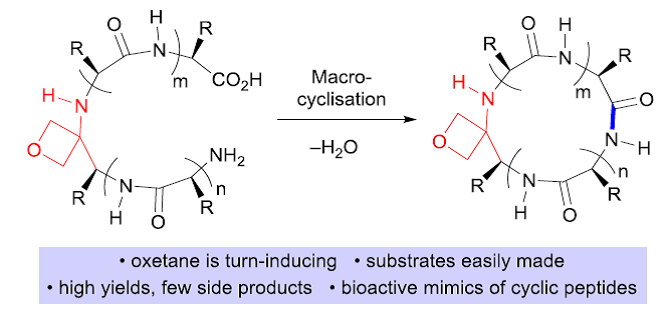
• A. Noble, S. Roesner, V. K. Aggarwal, Angew. Chem. Int. Ed. 2016, 55, 15920; Angew. Chem. 2016, 128, 16152. ‘Short Enantioselective Total Synthesis of Tatanan A and 3-epi-Tatanan A Using Assembly-Line Synthesis’. [Abstract]
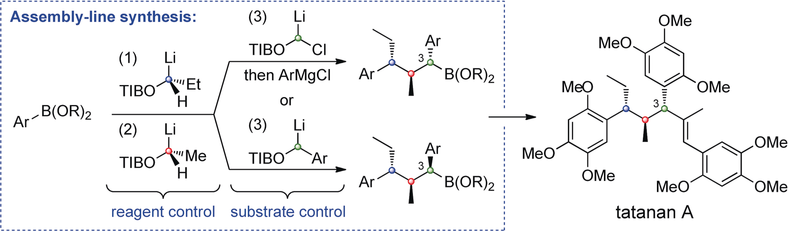
• S. Roesner, S. L. Buchwald, Angew. Chem. Int. Ed. 2016, 55, 10463; Angew. Chem. 2016, 128, 10619. ‘Continuous-Flow Synthesis of Biaryls by Negishi Cross-Coupling of Fluoro- and Trifluoromethyl-Substituted (Hetero)arenes’. [Abstract]
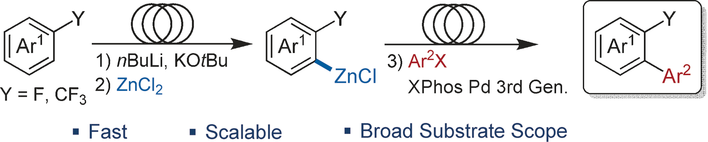
• S. Roesner, D. J. Blair, V. K. Aggarwal, Chem. Sci. 2015, 6, 3718. ‘Enantioselective Installation of Adjacent Tertiary Benzylic Stereocentres by Using Lithiation–Borylation–Protodeboronation Methodology. Application to the Synthesis of Bifluranol and Fluorohexestrol’. [Abstract]
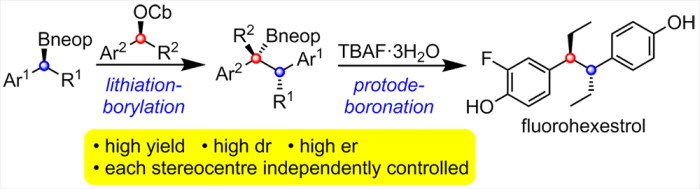
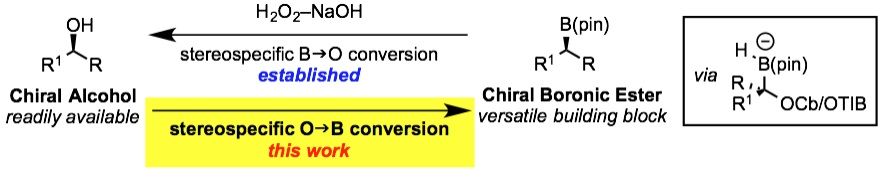
• S. Roesner, C. A. Brown, M. Mohiti, A. P. Pulis, R. Rasappan, D. J. Blair, S. Essafi, D. Leonori, V. K. Aggarwal, Chem. Commun. 2014, 50, 4053. ‘Stereospecific Conversion of Alcohols into Pinacol Boronic Esters Using Lithiation‒Borylation Methodology with Pinacolborane’. [Abstract]
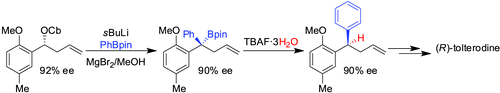
• S. Roesner, V. K. Aggarwal, Can. J. Chem. 2012, 90, 965. ‘Enantioselective Synthesis of (R)-Tolterodine Using Lithiation/Borylation Methodology’. [Abstract]
• S. Roesner, V. K. Aggarwal, Nature 2012, 487, 48. ‘Organic Chemistry ‒ Reactions at the End of a Tether’. ‘News and Views’ article.

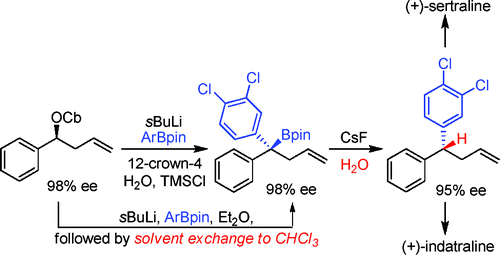
• S. Roesner, J. M. Casatejada, T. G. Elford, R. P. Sonawane, V. K. Aggarwal, Org. Lett. 2011, 13, 5740. ‘Enantioselective Syntheses of (+)-Sertraline and (+)-Indatraline Using Lithiation/Borylation Methodology’. [Abstract]
• S. Roesner, G. Hilt, Synthesis 2011, 662. ‘Substrate-Controlled Regioselective Cobalt(I)-Catalysed 1,4-Hydrovinylation Reactions’. [Abstract]
• L. Kersten, S. Roesner, G. Hilt, Org. Lett. 2010, 12, 4920. ‘Synthesis and Characterization of Polycarbonyl Compounds via their BF2-Adducts’. [Abstract]