Falk Schneider
Summary
In the Fluorescence and Membrane Dynamics (FMD) Lab, we investigate how molecular motions and interactions give rise to complex organisation and function. Our research spans model systems of varying complexity. We work with artificial membranes with reconstituted proteins, cultured cells, and zebrafish embryos, enabling us to study dynamics across different biological scales. While in vitro systems provide a high degree of control, the physiological context of an organism offers unchallenged insights into the role of the native environment.
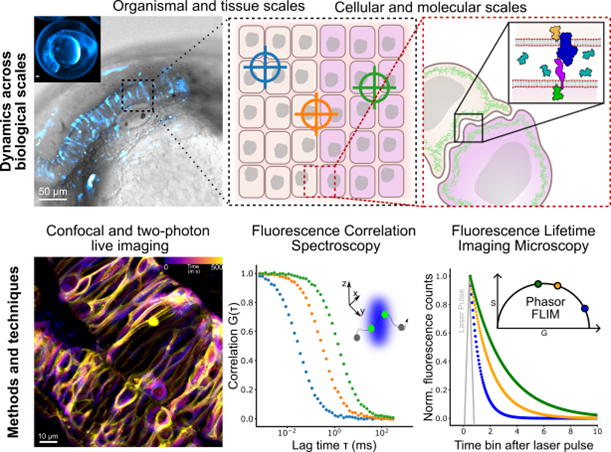
Our primary tools are based on advanced fluorescence microscopy. We develop and apply fluorescence correlation spectroscopy (FCS) and fluorescence lifetime imaging microscopy (FLIM) methods to make every photon count. Increasing sensitivity and specificity is especially important in deep tissue imaging. Currently, the lab focuses on studying the mechanisms underlying zebrafish hindbrain development, establishing methods to link nanometre-scale molecular dynamics, cellular signalling, and tissue-scale mechanics.
Key questions include:
1. How are lipid and receptor mobility in the plasma membrane influenced by tissue-scale mechanics?
2. How is the extracellular space organised, and how does it affect ligand delivery to membrane-bound receptors?
3. How are biophysical properties contributing to cellular identity within the tissue?
By bridging scales and developing cutting-edge fluorescence techniques and probes, the FMD Lab aims to uncover the fundamental biophysical organising principles that underpin complex biological systems and phenomena.
Selected publications:
Schneider F, Cespedes PF, Karedla N, Dustin ML, Fritzsche M. Quantifying biomolecular organisation in membranes with brightness-transit statistics, Nat Commun 15, 7082. 2024.
Luu P, Fraser SE, Schneider F. More than double the fun with two-photon excitation microscopy, Commun. Biol. 7, 364. 2024
Mørch AM, Schneider F, Jenkins E, Santos AM, Fraser SE, Davis SJ, Dustin ML. The kinase occupancy of T cell coreceptors reconsidered, PNAS 119 (49). 2022.
Schneider F, Sych T, Eggeling C, Sezgin E. Influence of nanobody binding on fluorescence emission, mobility, and organization of GFP-tagged proteins, iScience 24 1. 2021.
Schneider F, Hernandez-Varas P, Christoffer Lagerholm B, Shrestha D, Sezgin E, Julia Roberti M, Ossato G, Hecht F, Eggeling C, Urbančič I. High photon count rates improve the quality of super-resolution fluorescence fluctuation spectroscopy, J Phys D Appl Phys 53 164003. 2020.
Schneider F, Waithe D, Lagerholm BC, Shrestha D, Sezgin E, Eggeling C, Fritzsche M. Statistical Analysis of Scanning Fluorescence Correlation Spectroscopy Data Differentiates Free from Hindered Diffusion, ACS Nano 12 8. 2018.
Schneider F, Waithe D, Galiani S, Bernardino de la Serna J, Sezgin E, Eggeling C. Nanoscale Spatiotemporal Diffusion Modes Measured by Simultaneous Confocal and Stimulated Emission Depletion Nanoscopy Imaging, Nano Lett 18 7. 2018.
Schneider F, Waithe D, Clausen MP, Galiani S, Koller T, Ozhan G, Eggeling C, Sezgin E. Diffusion of lipids and GPI-anchored proteins in actin-free plasma membrane vesicles measured by STED-FCS, Mol Biol Cell 28 11. 2017.

