A complete up-to-date list of publications of Robert can be found on Google Scholar and full-texts might be available from the Warwick Research Active Portal (WRAP).
 |
Recent clinical results suggest that early immunotherapy is more efficacious than late immunotherapy. In a commentary lead by Seline Ismail-Sutton, we argue that "Personalising chronotherapy of immune checkpoint blockade" is important. Chronotype as a factor should be looked at in pragmatic chrono-immunotherapy trials. |
 |
Now out as paper in Biomacromolecules. Led by Sophie Laroque from the Perrier Lab in Warwick and in collaboration with Katherine Locock at CSIRO Australia, we show the anti-inflammatory effects of novel cationic co-polymers that also show anti-microbial activity. Win-win for sepsis patients. |
New paper by Sophie Hill reporting on her drug delivery work during her PhD in the Perrier lab.
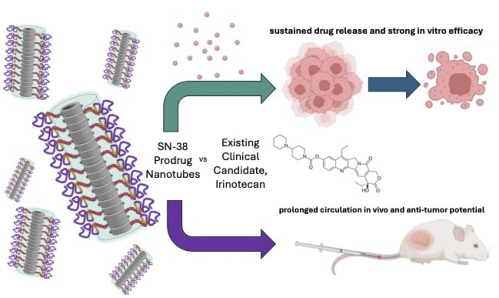 |
Cyclic peptide-polymer conjugate nanotubes have been shown to be powerful drug delivery vectors, due to their propensity for dynamic self-assembly, high aspect ratio morphology and structural interchangeability. Building upon previous studies that demonstrate the shielding abilities of the polymeric corona of nanotubes to enhance pro-drug bond stabilities and modulate hydrolysis, we utilise here the concept of a hydrophobic core building block with multiple drug units to improve drug loading capacity and overall efficiency of the nanotube carriers. By leveraging the intermolecular features of the drug core to strengthen assembly, we hypothesise that these nanotubes have the potential as a responsive supramolecular delivery system whereby upon full hydrolysis of the labile drug, these core forming interactions disappear, and nanotubes can fall apart and undergo clearance. Herein, we explore the self-assembly, in vitro efficacy and in vivo pharmacokinetic and anti-tumour pharmacodynamics of these nanotubes in colorectal cancer models, comparing the potent topoisomerase inhibitor SN-38 with its clinically-used parent pro-drug irinotecan. |
Great collaboration with Seb Perrier and Gabriele Sosso from Warwick Chemistry. Vito's first paper from his PhD work in the IBR MRCDTP.
 |
Crossing the blood-brain barrier (BBB) remains a major obstacle for central nervous system therapeutics. Short peptides have emerged as promising vectors, including cell-penetrating peptides (CPPs) and brain-penetrating peptides (BPPs). However, the structural and physicochemical features that distinguish CPPs from BPPs remain poorly understood, limiting rational design. Here, we systematically analysed their amino acid composition, sequence distribution, and physicochemical descriptors. Our findings suggest that BBB penetration is not a simple extension of cell penetration but requires finely tuned physicochemical properties. This study provides mechanistic insights into BPP design and highlights machine learning as a valuable tool for engineering next-generation BBB-penetrating peptides and peptide-mimetic materials. |
New study from Ed Lant's PhD project, entitled "Tuning Ligand Substituents for Enhanced Catalytic Activity and Antiproliferative Effects in Rh(III) Azopyridine Complexes".
 |
We report the synthesis and characterisation of ten novel half-sandwich Rh(III) azopyridine complexes as potential anticancer agents, with the general formula [Rh(η5-Cpx)(4-R2-phenylazopy-5-R1)Cl]PF6, where Cpx = Cp*, CpxPh or CpxPhPh, R1 = H, Br, or CF3, and R2 = H, OH or NMe2. X-ray crystallographic data for complex 2 (R1 = Br, R2 = OH, Cpx = CpxPh) and complex 3 (R1 = CF3, R2 = OH, Cpx = CpxPh) confirm their typical half-sandwich “piano-stool” geometry. The substituents have a major effect on the cytotoxicity of these complexes towards human ovarian (A2780 and cisplatin-resistant A2780cis), lung (A549), prostate (PC-3) and breast (MCF-7) cancer cells, and non-cancerous human lung fibroblasts (MRC5). Potencies range from sub-micromolar to inactive within the concentration range investigated (<100 µM). Selectivity for cancer cells over non-cancerous cell is strongly dependent on the nature of the bidentate ligand. The nano-molar active, highly lipophilic complex 2 was strongly accumulated by cells, and catalyzed the oxidation of NADH to NAD+, and GSH to GSSG. Notably, complex 2 is almost an order of magnitude less toxic in vivo than cisplatin. These complexes appear to have an unusual mechanism of anticancer activity, associated not only with Rh(III) but also the azo bond and activated cyclopentadienyl methyl groups. |





