Ceri Lyn-Adams
 |
 |
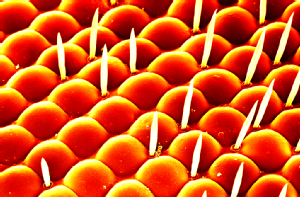
A 'guess what' moment |
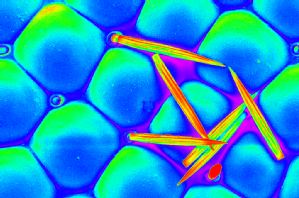
Carried away with colour |
What got you interested in this area of science?
My Grandfather died in the year 2000 as a result of a brain degenerative disease, which was diagnosed in the latter part of his life as Alzheimer’s. It was excruciatingly painful to witness such a proud man suffering the humiliation of losing his mind and cognitive functions. At times, he could become so confused he would believe he was back in the Second World War or at some other location buried in the memories of his early childhood. I was fourteen years old when my Grandfather died and I resolved that in the future I wanted to do something, anything, to help those suffering from such a terrible affliction. My early experience with Alzheimer’s disease shaped my life. What I witnessed, through my Grandfathers’ suffering moved me greatly and influenced my desire to become a research scientist and strive to make a difference in the field of Neuroscience and in particular neurodegenerative research.
How is the work you are currently doing relevant to everyday life?
Due to advances in healthcare and nutrition, humans are living longer. Although a welcome development, it does raise new issues that have to be addressed to make this longevity happy and healthy. Key amongst these issues is the fact that as the human brain ages it runs the risk of suffering cognitive decline and dementias such as Alzheimer's Disease (AD). Dementia UK estimate the total cost of dementia amounts to over £17 billion per annum to the UK alone. There is clearly a real social, economic and scientific need to move dementia research forward.
What does your research hope to achieve?
Studying age related diseases in humans presents problems as studies naturally have to be conducted over a long time scale. Therefore, there is no question over the requirement for animal models in research into age-related diseases. Clearly much of the work on the degeneration of higher brain function must be carried out in complex and ethically sensitive models such as rodents. However, we believe that much of the basic molecular work can be conducted effectively in lower animals. Using the fruit fly (Drosophila melanogaster), we are replacing some of the requirement for rodent models by studying the normal and abnormal function of proteins and their regulators known to be associated with the development and degeneration of the brain. We are establishing this system as a screen for new molecules and proteins that affect brain function, including drugs that may help to prevent some of the basic biochemical problems linked with AD. This approach will help to direct drug development programmes, meaning that only the most promising candidates will be tested in more complex models such as rodents.
One of the biochemical problems identified in AD is the interaction of proteins termed "kinases" and "tau". Tau is a protein responsible for one of the two diagnostic criteria of AD - the neurofibrillary tangle. These tangles accumulate inside brain nerve cells and promote their destruction. Understanding how this accumulation occurs is an important goal in AD research and may provide means by which to prevent, or even reverse, tangle formation.
My project, funded by the Alzheimer’s Research Trust, confirms that it is possible to study the interaction between human protein kinases and human tau in Drosophila. Our studies not only help characterise the molecular basis of the interaction that leads to tau-dependent damage but also moves toward the development of a screen for potential drug treatments that could reduce this damage.
what do the images show?
Beauty is in the eye of the beholder: A high resolution microscope image (scanning electron micrograph) of the head of a fruit fly. In my studies I use the eye of the fruit fly as an external read out of protein-protein interactions. There is a wealth of tools available for use in the fruit fly and one of these tools is the ability to express proteins in a particular location, in our case, specifically in the eye. As seen in this image, the structure of the wild type fruit fly eye is very organised and regular; expression of disease related proteins which can cause disruption to this structure gives us an insight into the interactions between the proteins.
Carried away with colour: A psuedo-coloured, high resolution microscope image (scanning electron micrograph) of individual lenses (ommatidia) in the fruit fly eye. The eye of the fruit fly contains 700-800 of these lenses, intersected with bristles which in this image have been broken. In my research I express disease related proteins in the fruit fly eye and look for disruption to the regular structure seen here.

