News Library
Tweakable chiral magnetic materials
Magnetic interactions between metal atoms in a family of chiral materials respond to subtle changes in their organic chemistry. The work forms part of the PhD research by Lihong Li in the Scott group in Warwick Chemistry department. Read the article at Inorg. Chem. 2011
Corkscrew Conductors
A new family of semiconductors in which the electricity is transported through nanoscopic helices have been reported by the Scott group at Warwick. The work forms part of the PhD research by former Warwick Postgraduate Research Fellow
 , Dr Nikola Chmel
, Dr Nikola Chmel
 . Read the paper at Inorg. Chem. 2011
. Read the paper at Inorg. Chem. 2011
Graphene oxide’s solubility disappears in the wash
|
Drs Rourke and Wilson’s team made their discovery when treating the graphene oxide with sodium hydroxide (NaOH) in an attempt to increase the usefulness of the oxygen containing functional groups believed to be bound to the graphene. Unfortunately it seemed to make things worse rather than better. Indeed at high enough concentrations of NaOH Dr Rourke was left with a black suspension. The Warwick led researchers recalled that it had been shown that oxidation debris adheres to carbon nanotubes but the weak nature of the connection of this oxidation debris to the carbon nanotubes meant that a wash with a base can simply remove the oxidative debris. Experiments showed that in that particular case oxidative debris was found to make up almost a quarter of the mass of the “oxidized carbon nanotubes”. The researchers felt a similar process maybe happening in the Graphene Oxide they were studying. The results may also help explain the inordinately high levels of oxygen people were claiming to find in graphene oxide. Chemists were already struggling to identify enough plausible carbon to oxygen bonds to accommodate the amounts of oxygen believed to form part of graphene oxide.
The remaining liquid was also dried to give a white powder that the Warwick researchers showed contained the “oxidative debris” or OD; the OD was shown to be made up exclusively of small, low molecular weight compounds (i.e. less than 100 atoms). The graphene oxide recovered from washing process formed about 64% of the mass of the “graphene oxide” at the start of the process. The recovered OD or oxidative debris formed at least 30% of the weight of the mass of the original “graphene oxide”. Drs Rourke and Wilson’s team believe this shows that much of the oxygen that was believed to be closely bonded to the carbon in the graphene oxide was actually not bonded at all but simply lying on top of the graphene sheets, loosely connected to them as “oxidative debris”. This oxidative debris contained a large quantity of oxygen that simply came out in the wash when the graphene oxide was treated with sodium hydroxide. This creates a significant proble Drs Rourke and Wilson say “Our results suggest that models for the structure of graphene oxide need revisiting. These results have important implications for the synthesis and application of chemically modified graphene particularly where direct covalent functionalization of the graphene lattice is required.” The paper entitled: The Real Graphene Oxide Revealed: Stripping the Oxidative Debris from the Graphene-like Sheets by Dr. Jonathan P. Rourke, Priyanka A. Pandey, Joseph J. Moore, Matthew Bates, Neil R Wilson (all of the University of Warwick), and Dr Ian A. Kinloch, Prof. Robert J. Young (The University of Manchester), has just been published in Angewandte Chemie DOI: 10.1002/anie.201007520. Notes for editors: The researchers thank Dave Hammond for help with thermogravimetric analysis (TGA), Lijiang Song for help with mass spectrometry, and Ajay Shukla for help with X-ray photoelectron spectroscopy (XPS), the Midlands Physics Alliance Graduate School for a scholarship. The TEM, TGA, and XPS instruments as well as the mass spectrometer used in this research were purchased with support from Advantage West Midlands (part funded by the European Regional Development Fund) as part of the Science City programme. For further information please contact: Dr Jonathan P. Rourke Peter Dunn, Head of Communications PR23 8th March 2011 |
Zirconium catalysts not dead, just resting.
Dormant polymerization catalysts are given a rude awakening by Giles Theaker, Peter Scott and Warwick Chemistry Alumnus Colin Morton at Infineum. The results indicate how many old catalysts, thought to be dead, are just in a dormant state. The results published in the American Chemical Society journal Macromolecules describe a new mechanism for polymerization of styrenes.
Scott Group detects chiral building blocks for new materials
Pairs of building blocks are shown using spectroscopic and electrochemical techniques to associate in solution before forming new types of charge transfer material relevant to the search for chiral conductors. The work forms part of the PhD research by former Warwick Postgraduate Research Fellow , Dr Nikola Chmel
, Dr Nikola Chmel .
.
read the paper: http://dx.doi.org/10.1039/C0DT01184C
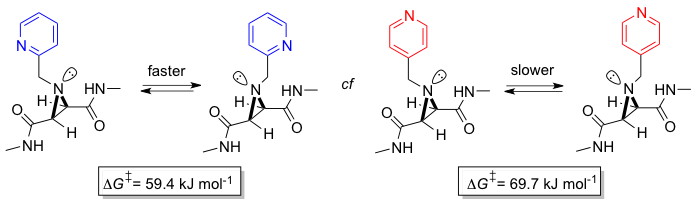
Shipman and Walsh groups report new method to quantify strength of hydrogen-bonds
Mike Shipman, Tiffany Walsh and co-workers have recently published a new method for detecting and quantifying noncovalent interactions. They have discovered that the rate of nitrogen inversion in aziridine derivatives is dependent on intramolecular interactions between attached functional groups. For example, the ortho-substituted pyridine undergoes faster inversion than its para-substituted analogue as a result of the formation of an intramolecular amide–pyridine (NH⋅⋅⋅N) hydrogen bond in the transition state (see graphic). Using simple NMR methods, it is possible to quantify the strength of these interactions in the transition state, and compare them with those predicted using computational methods. This work is expected to have applicability to a range of other important noncovalent interactions. It was conducted in collaboration with the Tucker group at the University of Birmingham.
The paper is published in the 17 January 2011 issue of Angewandte Chemie.
http://onlinelibrary.wiley.com/doi/10.1002/anie.201005580/abstract
Platinum and Blue Light Combine to Combat Cancer
When it comes to health care blue lights, are usually most useful on the top of ambulances but now new research led by the University of Warwick has found a way to use blue light to activate what could be a highly potent platinum-based cancer treatment.
Research led by the University of Warwick, along with researchers from Ninewells Hospital Dundee, and the University of Edinburgh, have found a new light-activated platinum-based compound that is up to 80 times more powerful than other platinum-based anti-cancer drugs and which can use “light activation” to kill cancer cells in a much more targeted way than similar treatments.
The University of Warwick team had already found a platinum-based compound that they could activate with ultra-violet light but that narrow wave length of light would have limited its use. Their latest breakthrough has discovered a new platinum based compound known as trans,trans,trans-[Pt(N3)2(OH)2(py)2] that can be activated by normal visible blue, or even green, light. It is also stable and easy to work with, and it is water soluble so it can simply dissolve and be flushed out of the body after use.
The University of Warwick researchers passed the new compound to colleagues at Ninewells Hospital Dundee, who tested it on oesophageal cancer cells cultivated within lab equipment. Those tests show that once activated by blue light the compound was highly effective requiring a concentration of just 8.4 micro moles per litre to kill 50% of the cancer cells. The researchers are also beginning to examine the compound’s effectiveness against ovarian and liver cancer cells. Early results there are also excellent but that testing work is not yet complete.
Professor Peter Sadler, from the Department of Chemistry from University of Warwick, who led the research project, said:
“This compound could have a significant impact on the effectiveness of future cancer treatments. Light activation provides this compound’s massive toxic power and also allows treatment to be targeted much more accurately against cancer cells.”
“The special thing about our complex is that it is not only activated by ultra-violet light, but also by low doses of blue or green light. Light activation generates a powerful cytotoxic compound that has proven to be significantly more effective than treatments such as cisplatin.”
We believe that photoactivated platinum complexes will make it possible to treat cancers that have previously not reacted to chemotherapy with platinum complexes,” says Sadler. “Tumors that have developed resistance to conventional platinum drugs could respond to these complexes and with less side-effects.”
This research has been supported by the EPSRC, MRC, ERC and Science City (ERDF/AWM).
Note for editors: The research has just been published in Angewandte Chemie, under the title “A Potent Trans Diimine Platinum Anticancer Complex Photoactivated by Visible Light”. The authors are – Project leader Professor Peter Sadler, (University of Warwick) and Nicola J. Farrer, Julie A. Woods, Luca Salassa, Yao Zhao, Kim S. Robinson, Guy Clarkson, and Fiona S. Mackay.
For more information please contact:
Professor Peter Sadler
University of Warwick, Department of Chemistry
Tel: +44 (0)7913 944357
P.J.Sadler@warwick.ac.uk
Peter Dunn, Head of Communications, University of Warwick,
44 (0)24 76 523708
mobile/cell +44 (0)7767 655860
p.j.dunn@warwick.ac.uk
PR171 9th December 2010
Mechanism of alkene hydroamination established by Peter Scott group and co-workers
A new class of highly active zirconium catalyst for the synthesis of chiral heterocycles from aminoalkenes is shown to operate via a Zr=N bonded (imido) species. Peter Scott and group members Laura Allan and Andrew Gott, in collaboration with David Fox and Guy Clarkson at Warwick, report in Journal of the American Chemical Society a detailed EPSRC-funded study which for the first time excludes the conventional Zr-N (amido) mechanism.
and group members Laura Allan and Andrew Gott, in collaboration with David Fox and Guy Clarkson at Warwick, report in Journal of the American Chemical Society a detailed EPSRC-funded study which for the first time excludes the conventional Zr-N (amido) mechanism.

Dr Manuela Tosin joins as assistant prof. of organic chemistry
Dr Manuela Tosin will be joining Warwick Chemistry as an Assistant Professor in Organic Chemistry from 1 November 2010. Dr Tosin's research interests are primarily in the area of the discovery and generation of new natural products. Manuela comes to Warwick Chemistry from the Department of Biochemistry, University of Cambridge where she worked with Dr Joe Spencer and Prof Peter Leadlay.
O’Reilly group reports on Multistep DNA-Templated Reactions
Phillip Milnes and Rachel O’Reilly collaborated with colleagues at the University of Oxford (Turberfield group) and University of Southampton (Stulz group) to report on Multistep DNA-templated reactions, which allow the synthesis of functional oligomers of an arbitrary length. Key features of the mechanism are that successive coupling reactions take place in near-identical environments and that purification is only necessary in the last synthesis step.
Angew. Chem. Int. Ed, 2010, early view (DOI: 10.1002/anie.201002721)
http://onlinelibrary.wiley.com/doi/10.1002/anie.201002721/abstract
New publication: A Potent Trans-Diimine Platinum Anticancer Complex Photoactivated by Visible Light
A Potent Trans-Diimine Platinum Anticancer Complex Photoactivated by Visible Light
N. J. Farrer, J. A. Woods, L. Salassa, Y. Zhao, K. S. Robinson, G. Clarkson, F. S. Mackay, P. J. Sadler
Angew. Chem. Int. Ed, 2010, early view. DOI: 10.1002/anie.201003399
Activating platinum with light: An inert platinum(IV) diazido complex trans,trantrans,trans-[Pt(N3)2(OH)2(py)2] becomes potently cytotoxic to cancer cells when activated by low doses of visible light.
http://0-www3.interscience.wiley.com.pugwash.lib.warwick.ac.uk/cgi-bin/fulltext/123597042/PDFSTART
Platinum(IV) complexes isomerising via agostic intermediates
Sarah Crosby, working in Jon Rourke's group, has identified a number of new Pt(IV) complexes containg dmso ligands.
Oxidation of cyclometalated Pt(II) complexes with S-bound DMSO ligands initially results in Pt(IV) complexes which retain the S-bound DMSO ligands in the same relative position. Isomerisation reactions result in a rearrangement of the ligands to give O-bound DMSO complexes, with the DMSO trans to a cyclometalated carbon. X-ray structures representing the only two known examples of Pt(IV) complexes with O-bound DMSO ligands have been solved. The rate of isomerisation of complexes without a pendant alkyl chain is strongly solvent dependent, consistent with the need to stabilise a coordinatively unsaturated intermediate. Pt(IV) complexes with a pendant alkyl chain show little dependence on isomerisation rate with solvent, with solution NMR data strongly suggesting the presence of agostic complexes. DFT calculations provide support for the presence of agostic complexes, with the same interactions being used to account for the loss of DMSO from the O-bound DMSO complexes.



