Histidines in metallothioneins
Previously characterised metallothioneins from yeast, vertebrates, and other animals coordinate metal ions exclusively through thiolate sulfurs. Up until fairly recently, this was a paradigm of metallothioneins. However, quite a number of metallothioneins do contain histidine residues, which may in principle also coordinate metal ions through their imidazole nitrogens (see: Histidines in metallothioneins (JIB 2008) ).
).
We have currently several metallothioneins under study in our lab in which histidine residues play pivotal roles in modulating the metal-binding properties of these intriguing residues.
- Bacterial metallothioneins homologous to SmtA contain at least one Cys3His site. The His residue in these appears to be vital for an ordered structure in the C-terminal part of these proteins (see: Role of histidines in bacterial MTs (JBIC 2007)
 )
)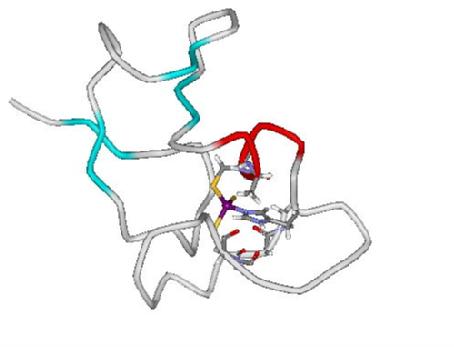
Picture based on pdb 1jjd (Blindauer et al., PNAS 2001).
- Type 4 plant MTs, for which wheat EC serves as the prototype, contain an isolated Cys2His2 site which is required for an ordered structure in the presence of zinc, but not cadmium: Role of histidines in discriminating between Zn and Cd in a plant MT (Mol. BioSyst. 2010). See also:
 Histidines in a plant MT (Proteins 2007)
Histidines in a plant MT (Proteins 2007)
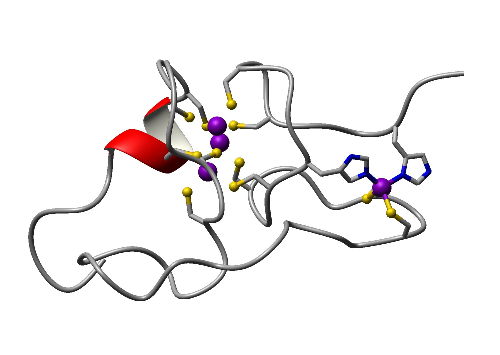
Picture based on pdb 2kak (Peroza et al., J. Mol. Biol. 2009)
- The two metallothioneins from the nematode C. elegans differ in their C-terminal parts by the presence of three additional His and one additional Cys residue in CeMT1. See paper The two C. elegans metallothioneins (CeMT1 and CeMT2) discriminate between essential zinc and toxic cadmium (FEBS J. 2010)
 , in which our collaborators and we show that the two isoforms contribute to discrimination between toxic cadmium and essential zinc. In Tools for metal ion sorting (Chem. Commun. 2011) we show that the two isoforms can partition Zn and Cd in vitro.
, in which our collaborators and we show that the two isoforms contribute to discrimination between toxic cadmium and essential zinc. In Tools for metal ion sorting (Chem. Commun. 2011) we show that the two isoforms can partition Zn and Cd in vitro.
