Orange Experiment
Investigation of an Esterase Enzyme from Orange Peel
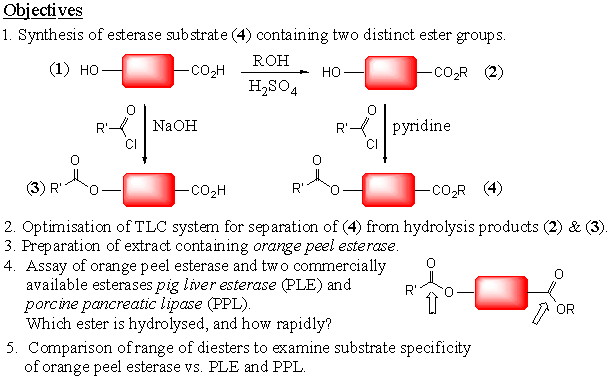
Enzymes are biological macromolecules that catalyse all of the chemical reactions that take place in biological systems. They are typically of molecular weight 10,000-100,000, and catalyse a specific chemical reaction with very high regio- and enantio-specificity. As well as being of academic interest due to their ability to catalyse complex chemical reactions under very mild conditions, they are important medicinal targets for antibiotics and chemotherapy. They are also increasingly used as reagents for organic synthesis, since in many cases they can catalyse large scale enantiospecific "bio-transformations".The idea of this practical experiment is to illustrate some features of enzyme catalysis, using a series of simple di-ester substrates that you will synthesise, and using an esterase enzyme which you will prepare as an extract from orange peel. It will also involve the use of thin layer chromatography as a means to separate the substrate and product of the reaction and hence as a means to assay the enzymatic reaction versus time. The experiment is designed to show that:
|
 |
|
Acid A = para-hydroxybenzoic acid |
