Teaching animations
I am learning to make animations as a hobby and here is a collection of some clips that I use in my teaching. Please feel free to download them for your own use and just acknowledge me (a right click and save the GIF should work). I welcome gentle corrections or suggestions for new content.
Please note that my target audience are undergraduate biochemists or the general public, not advanced mass spectrometrists, so I'm just trying to convey broad concepts as a quick introduction. Obviously my cartoon world has a great many simplifications, but I believe all leaning starts with a conceptual model which can then be refined with further reading and experience. There are very many resources available to you for further information but I can't list them here as I do not intend to provide a comprehensive course! Google, mass spec societies or your local lecturers are all reasonable starting points.
Analogy of Time-of-Flight mass spectrometry with ping pong balls
Apologies for the background noise. I'll re-shoot this soon. I just turn the sound off when using it.
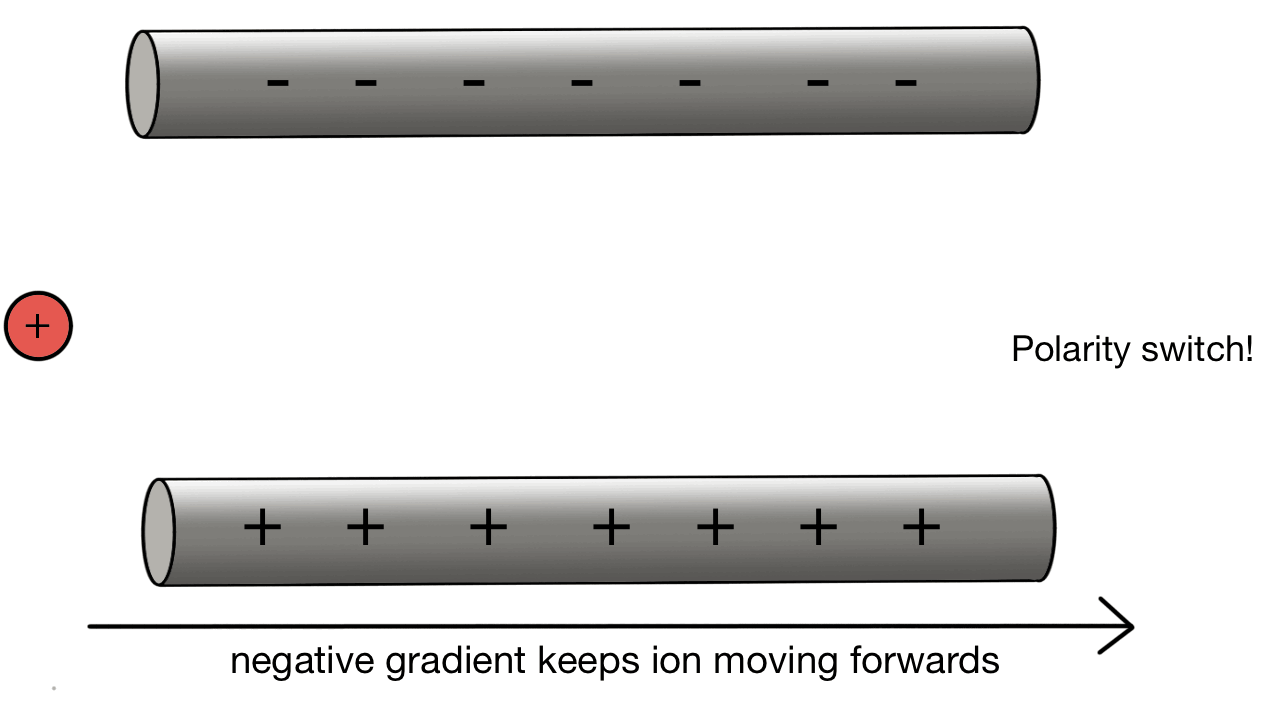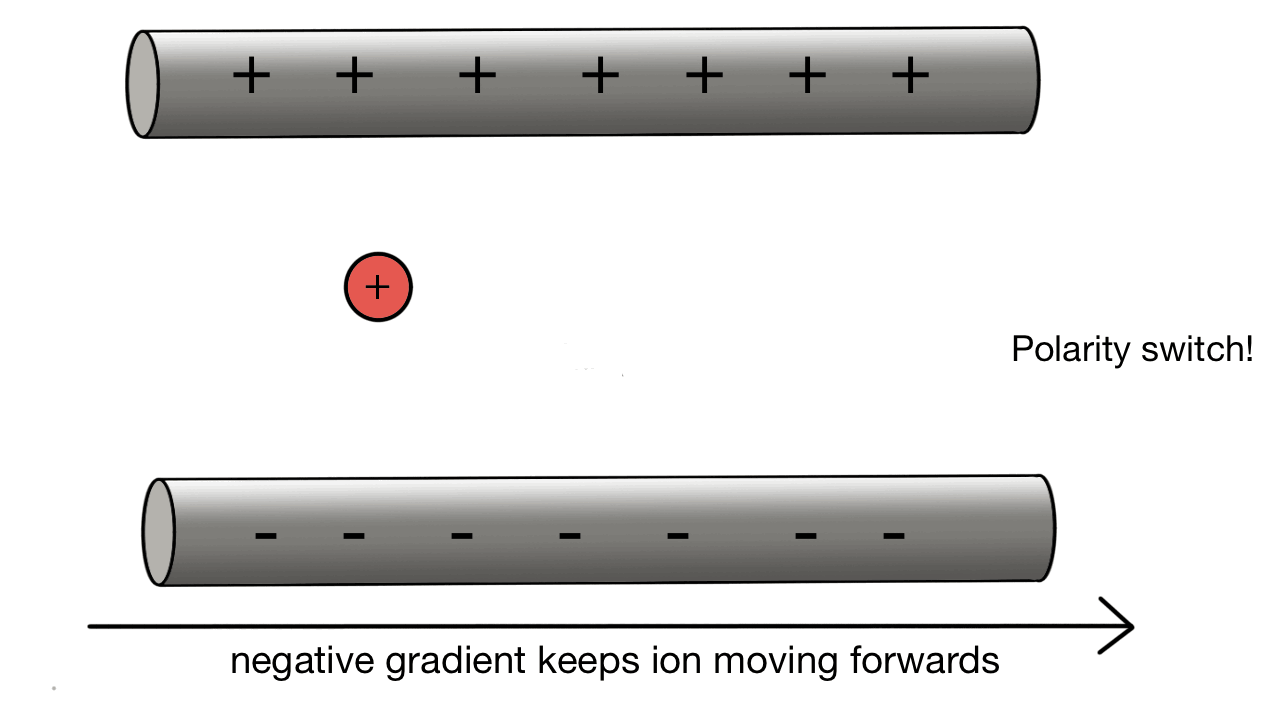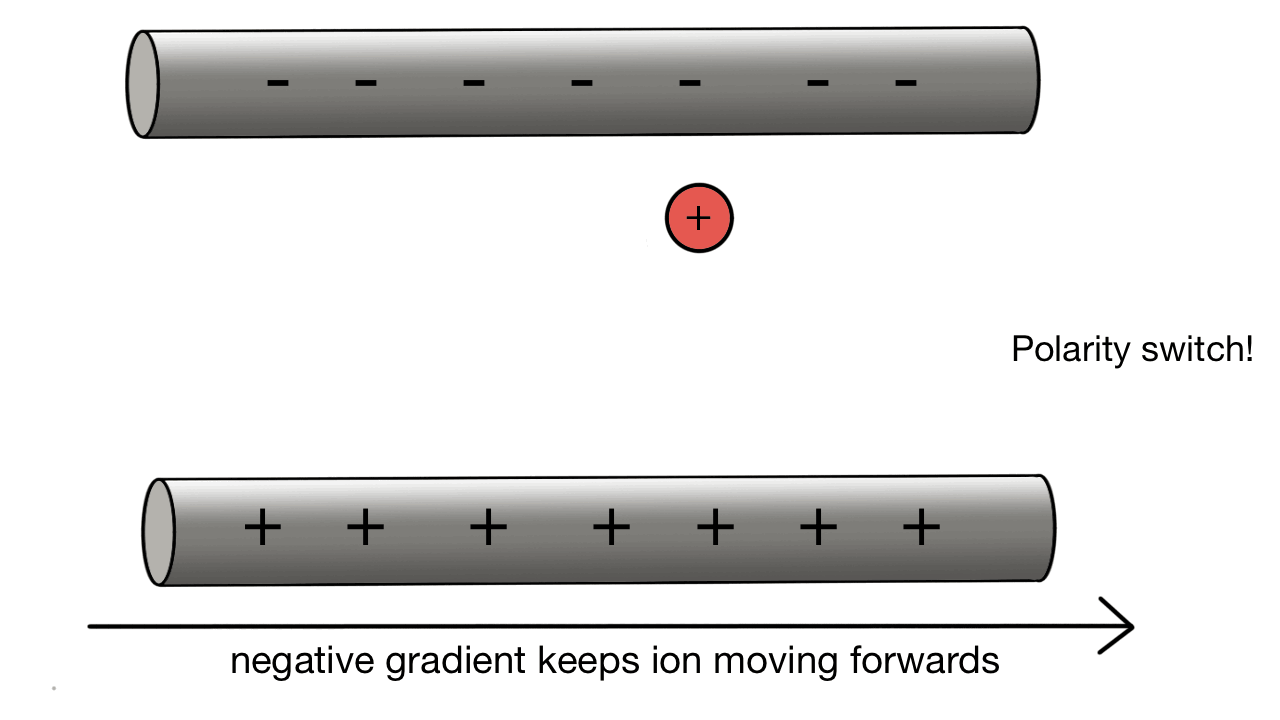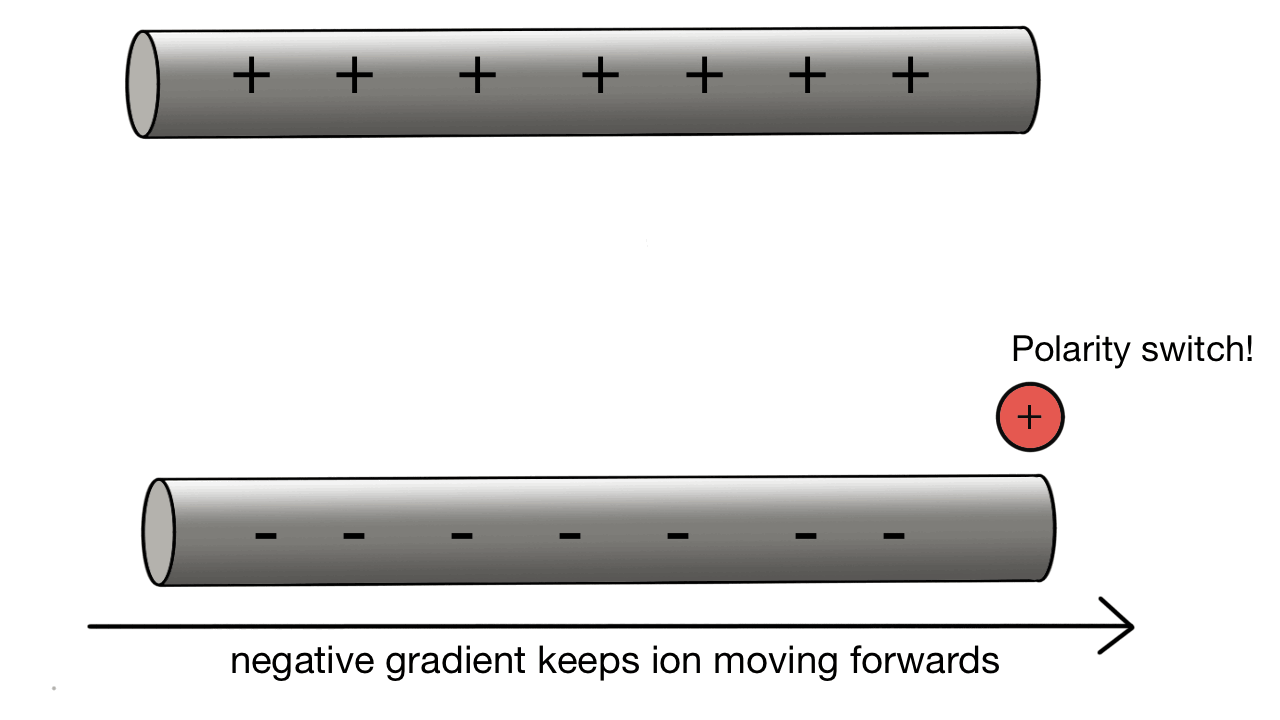
Quadrupole collection:
A positive ion would be attracted to a negatively charged pole.
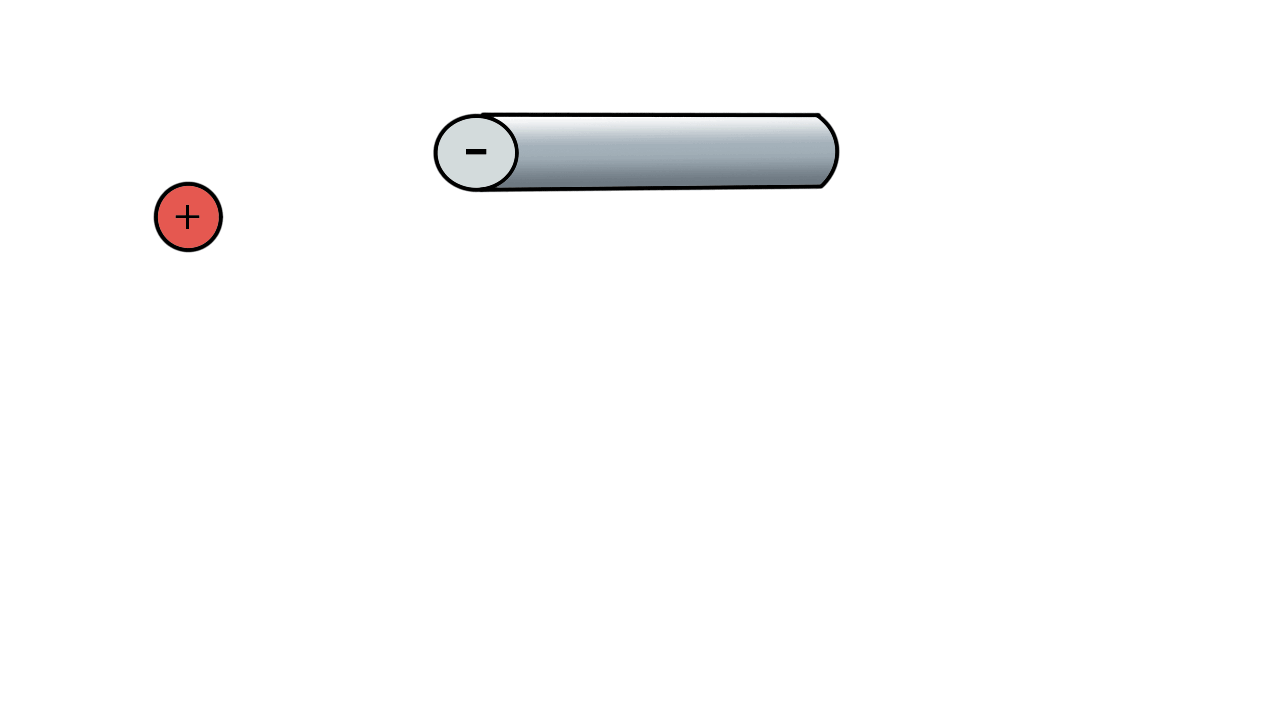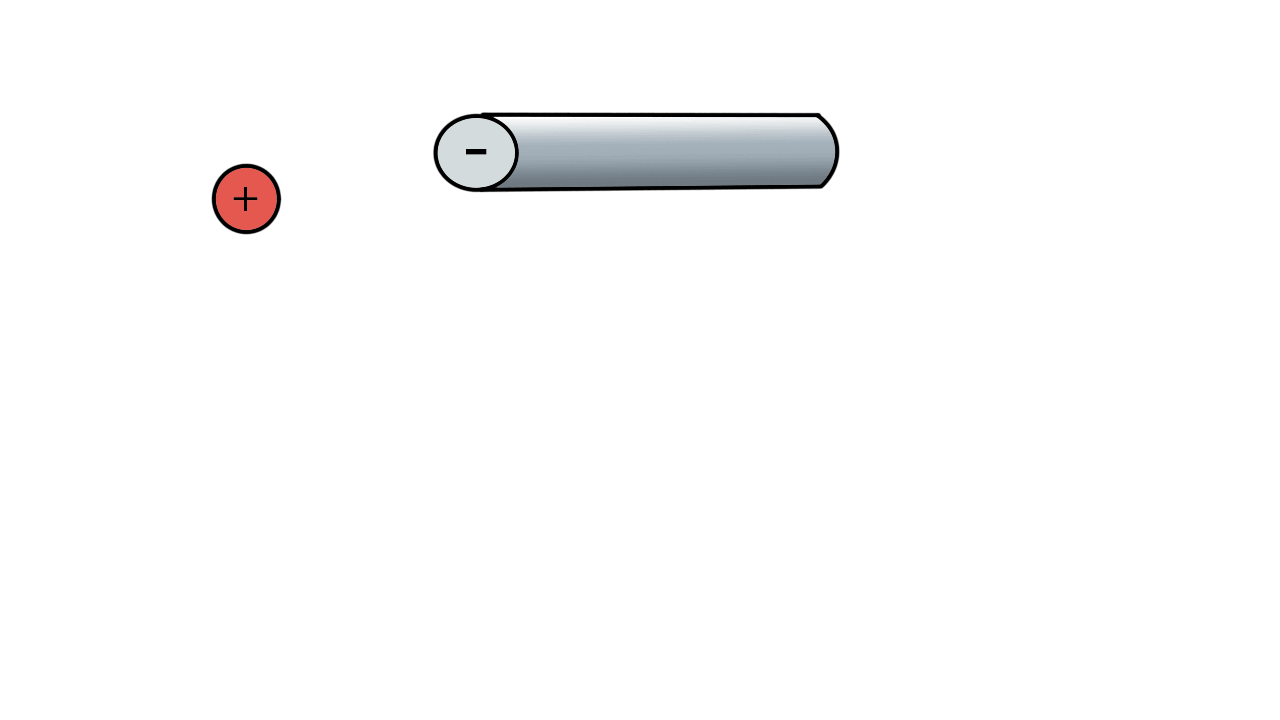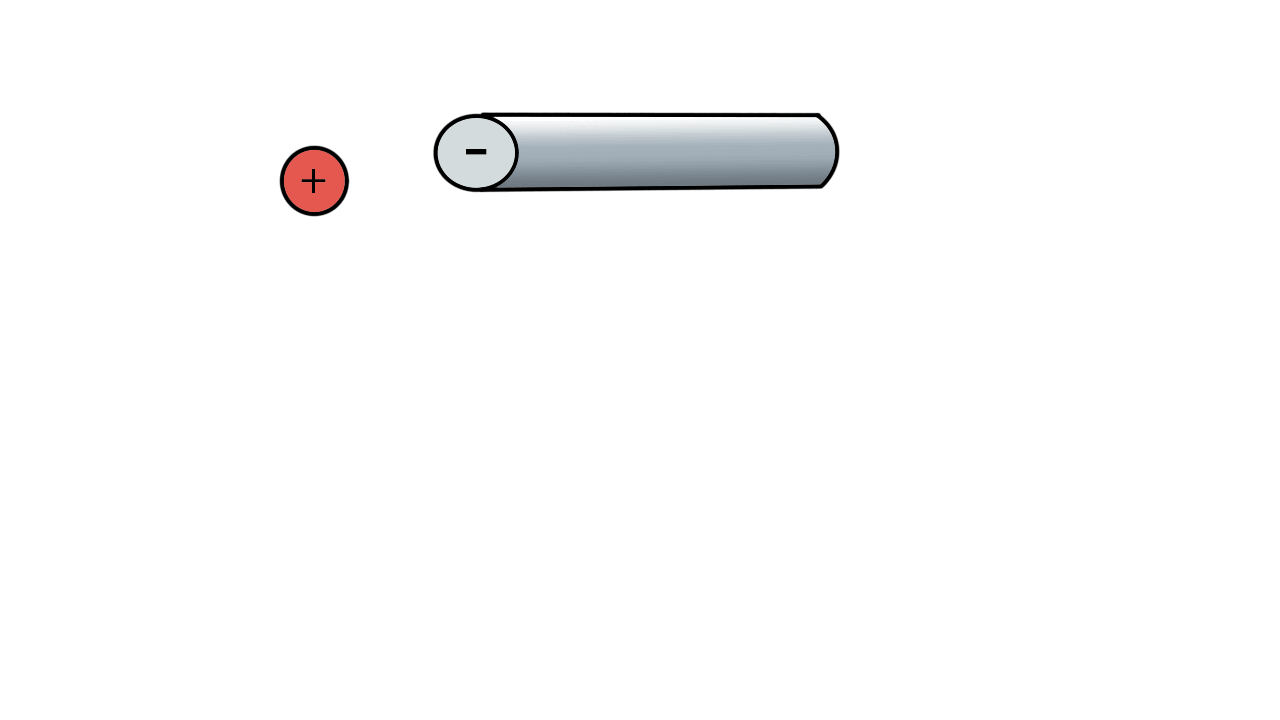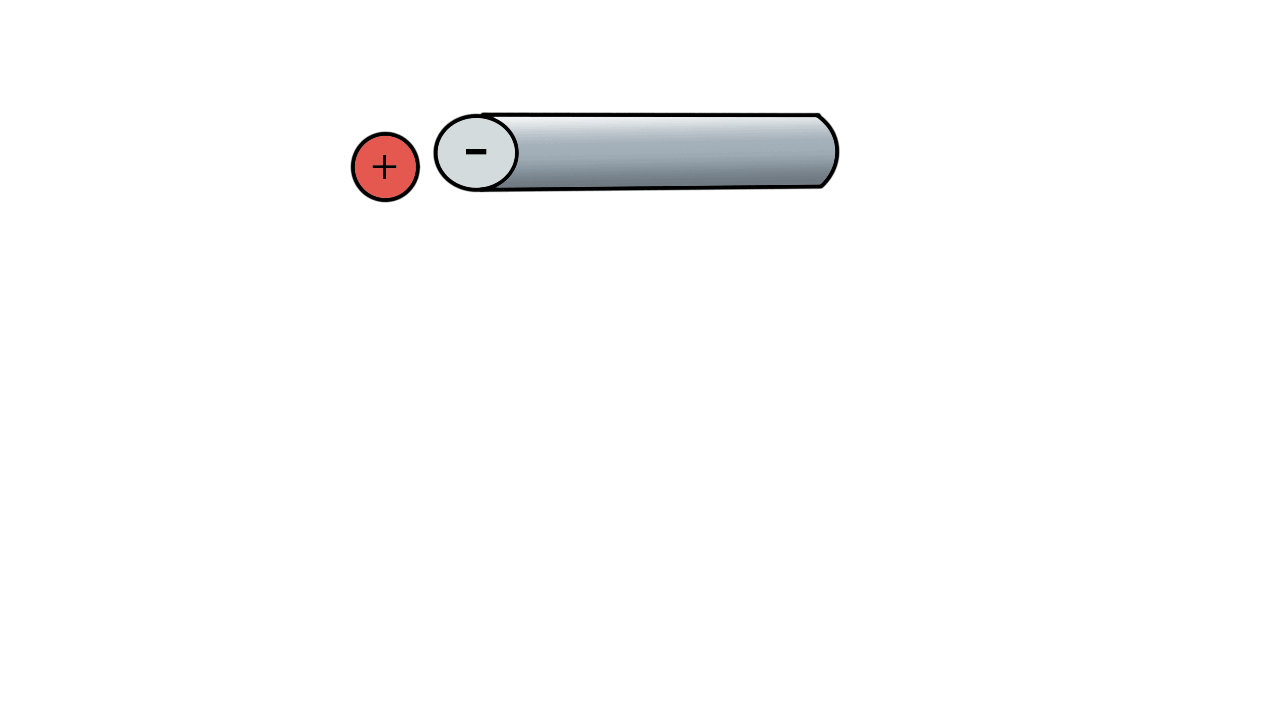
If we have two poles and switch polarity as the ion approaches, then we control how the ion moves.
But we need to control how an ion moves in 3D space. Four poles work well for this; an ion of a given mass to charge ratio (m/z) will have a stable path through the quad under specific conditions. In reality, the RF frequency is held constant and it is the RF and DC amplitudes that determine if a specific ion has a stable path in accordance with Mathieu's equation.
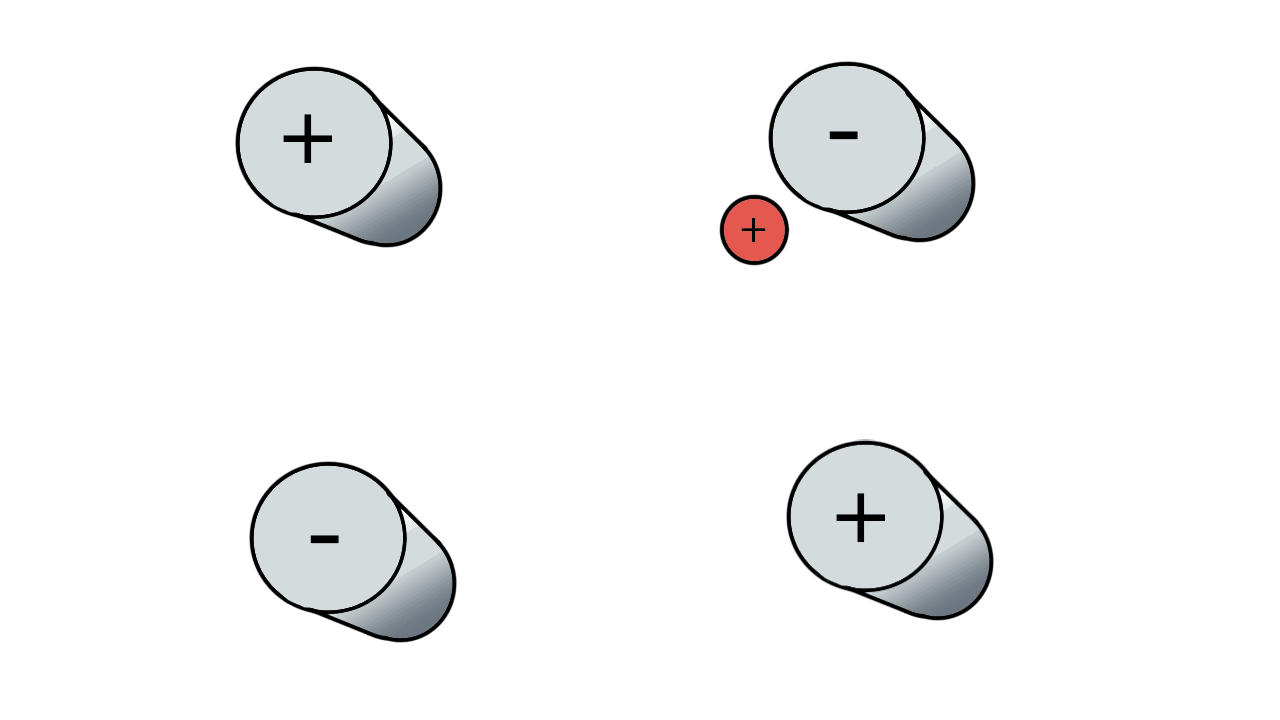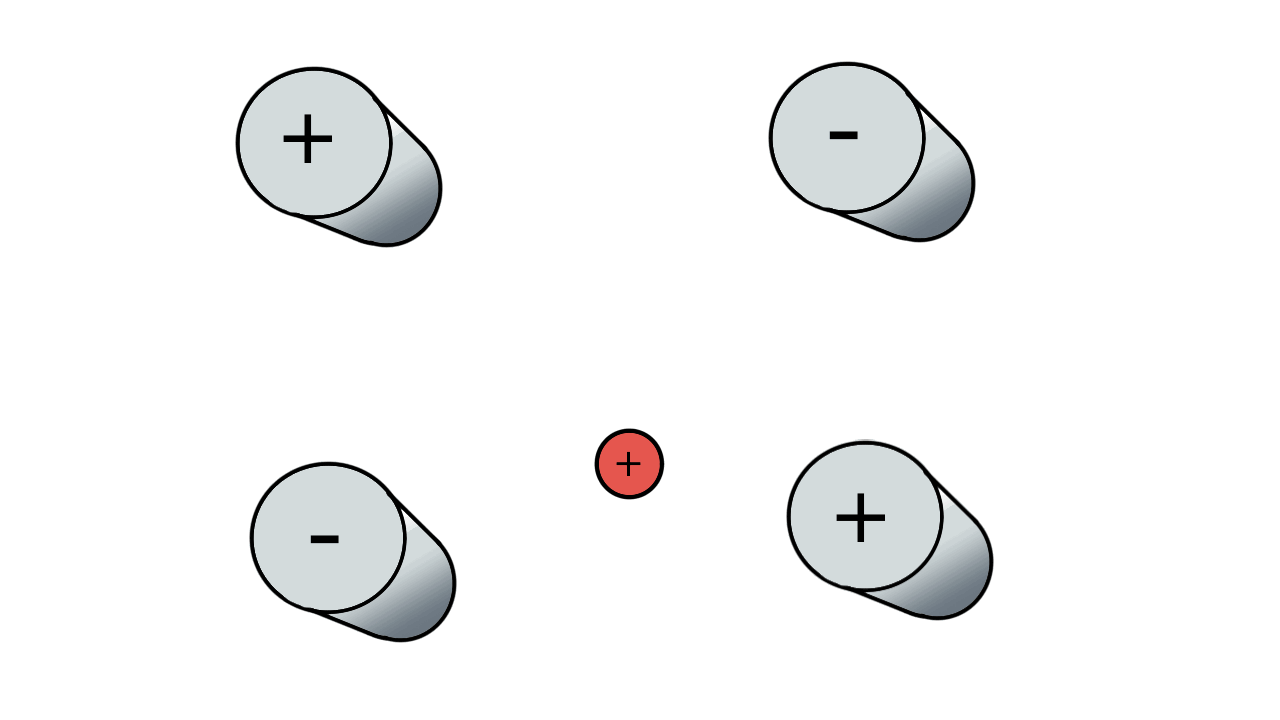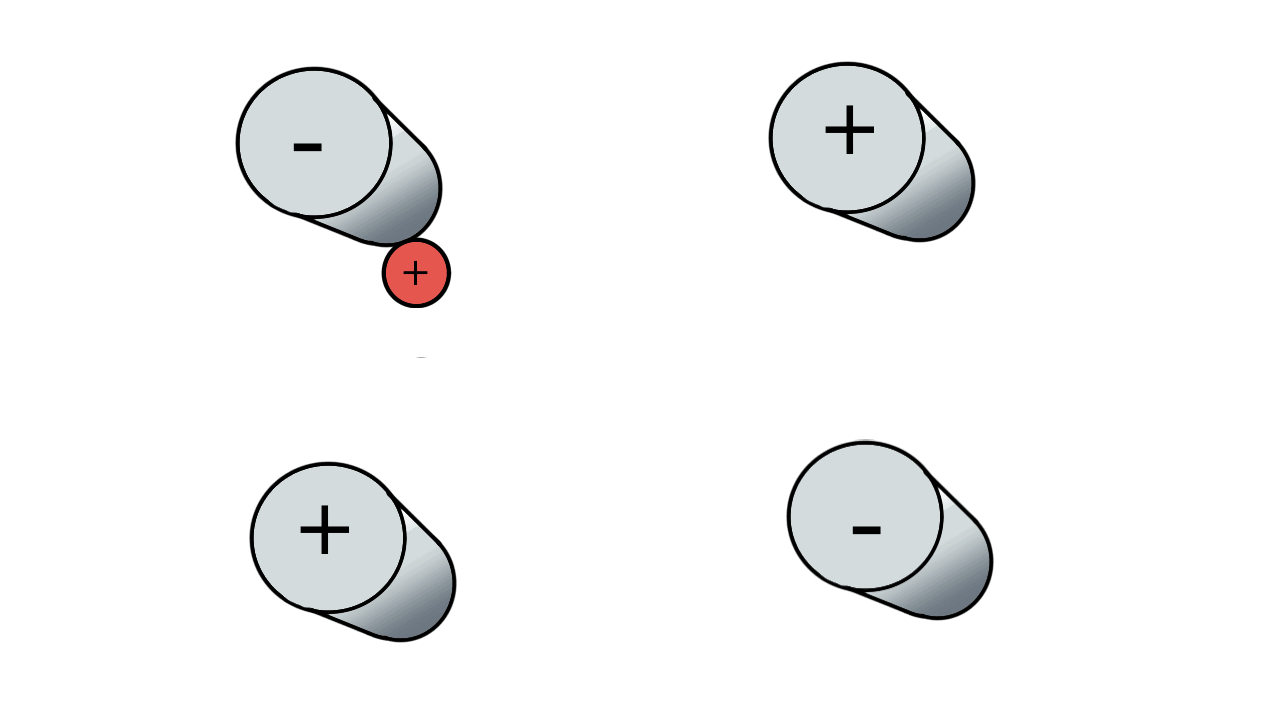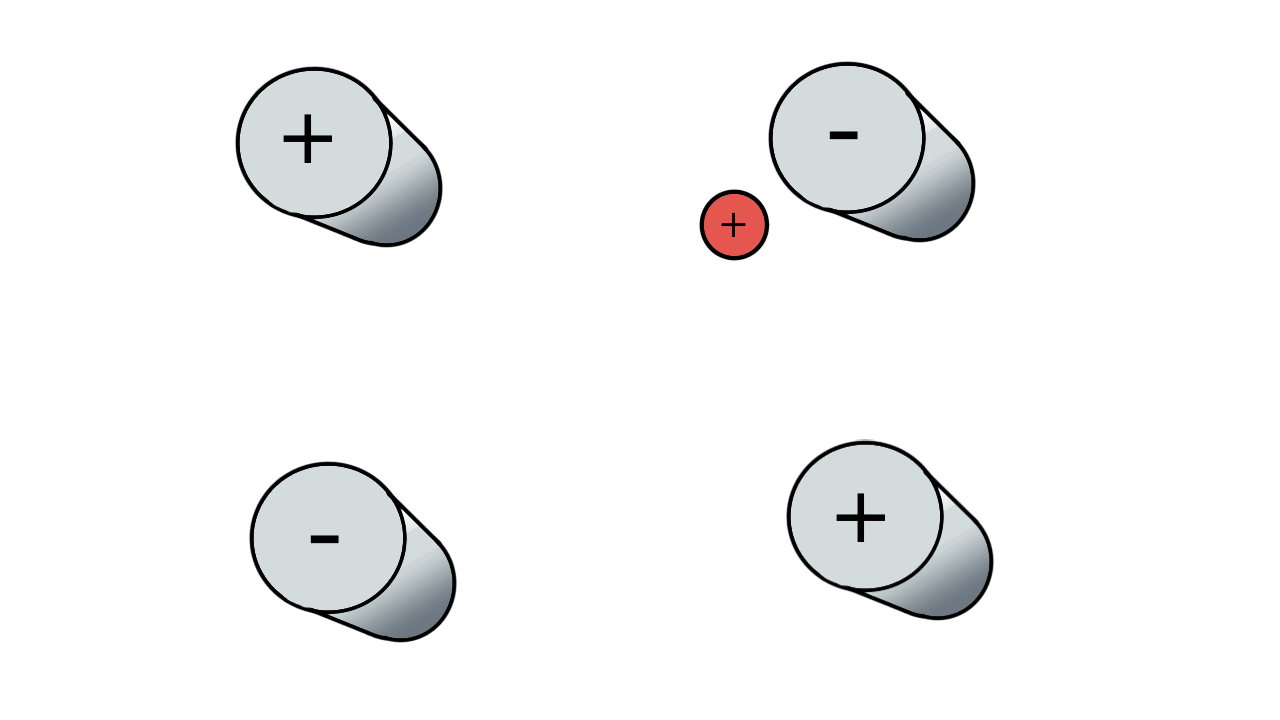
So if we have three ions of different m/z in the quadrupole, they will have different stable paths. Under the conditions below the red and green ions are stable and the big blue ion is lost from the quad.
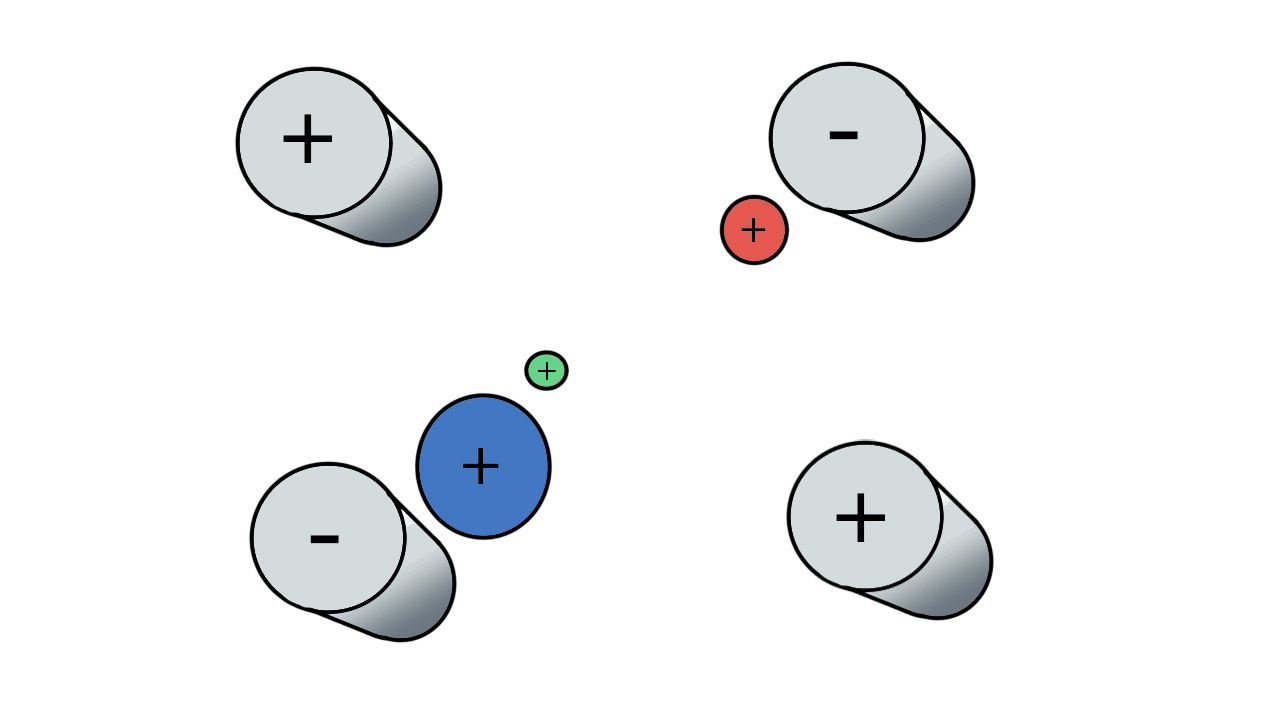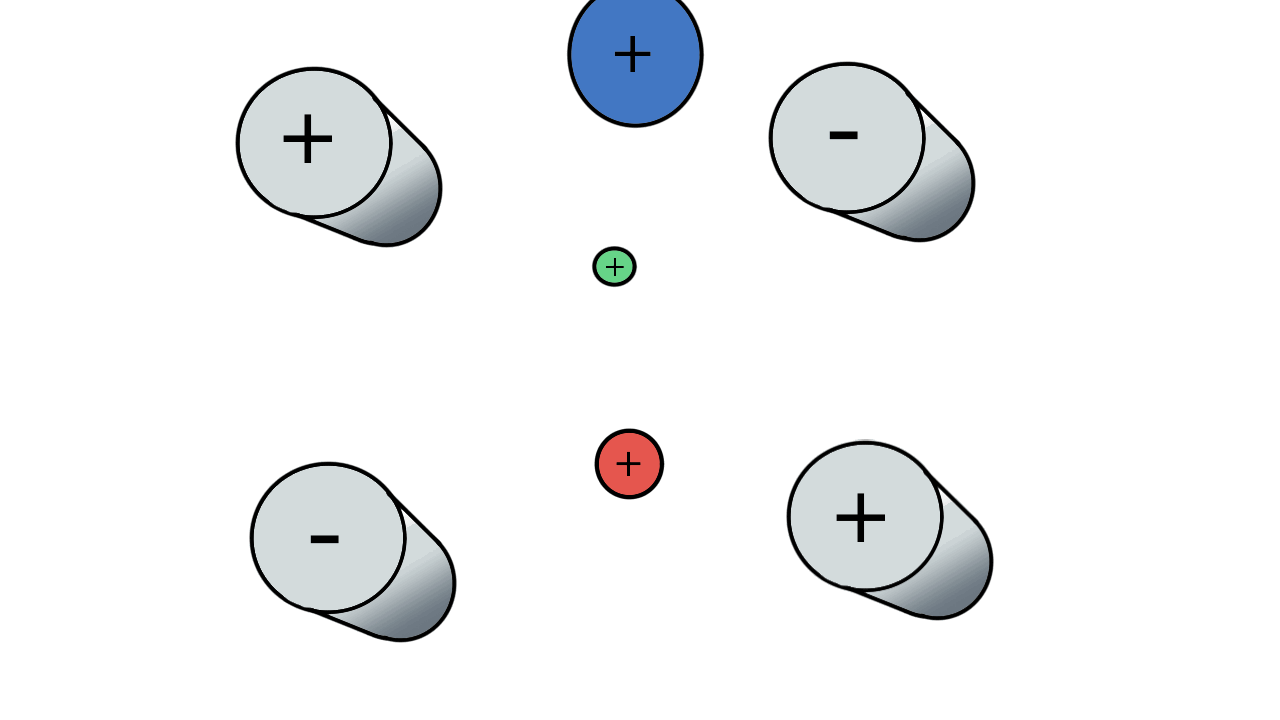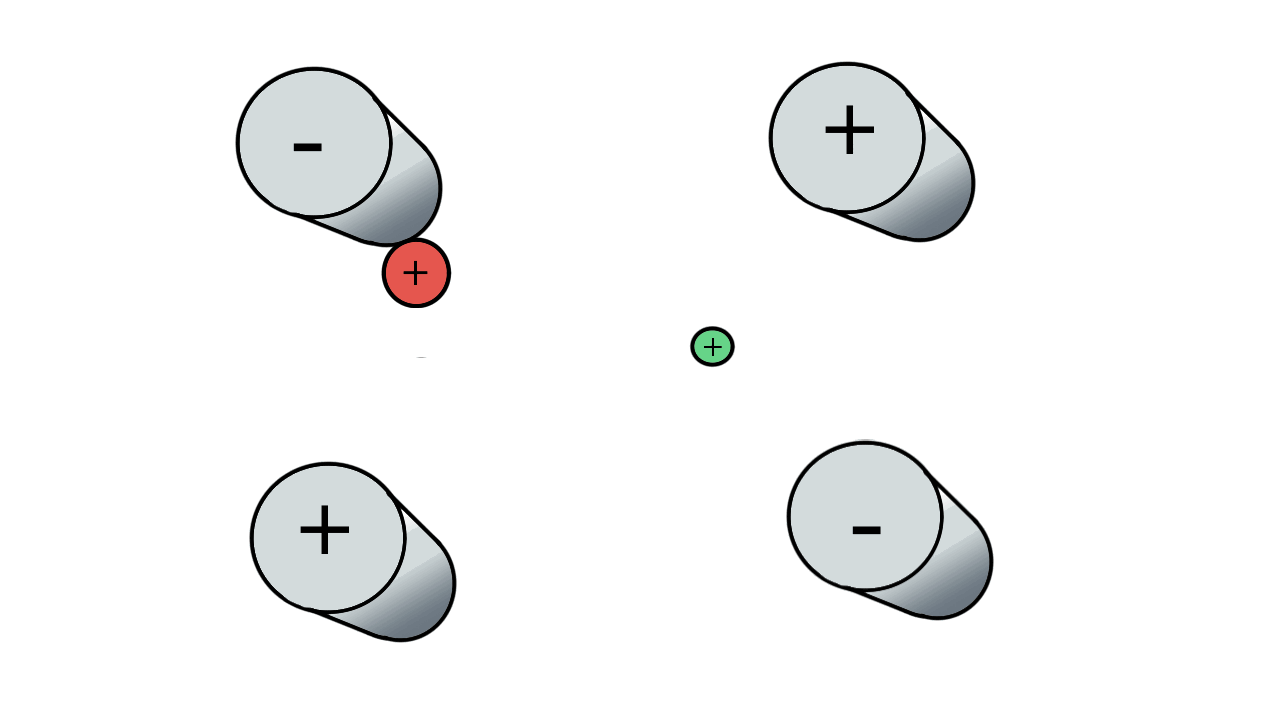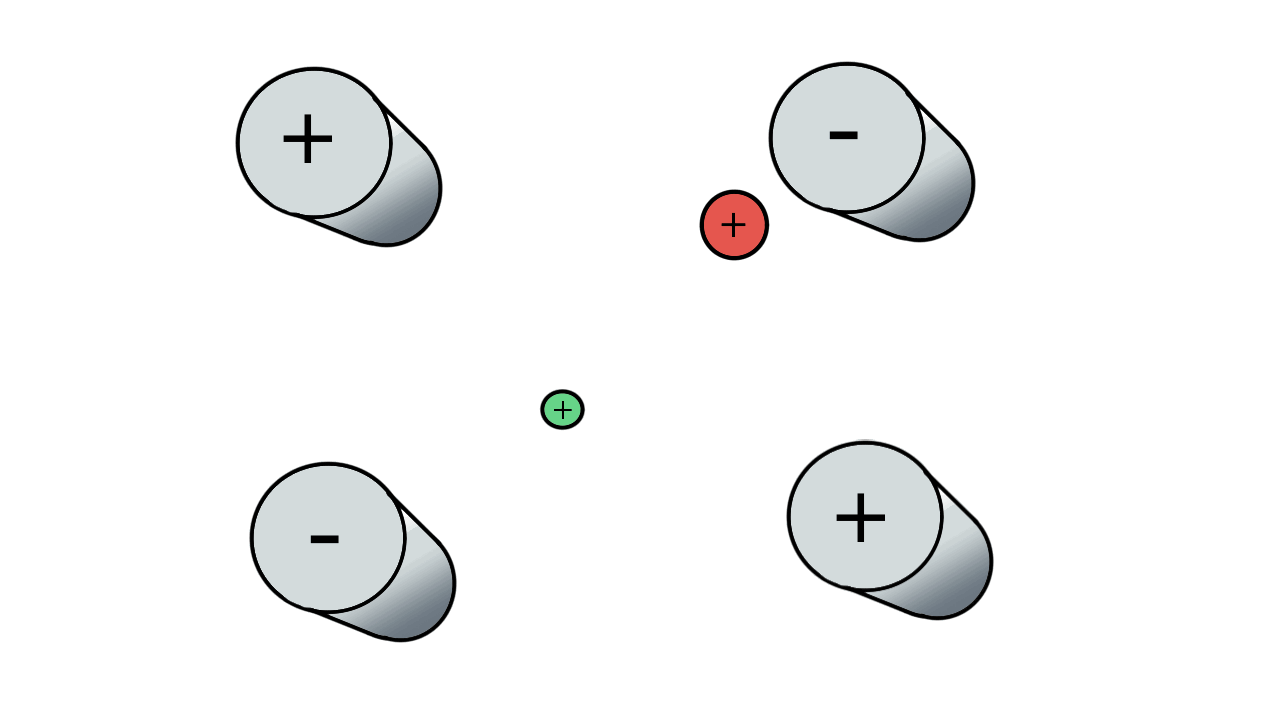
Remember that it is the mass to charge ratio (m/z) that determines how an ion moves in an electromagnetic field. So if we had that big blue ion present in three different charge states (+1, +2 and +3, represented in different shades of blue), then the 2+ ions would behave as if they had half the mass, and the 3+ ions would appear to have a third of the mass of the 1+ ion. (Note: a positive charge is usually conferred by the addition of a proton (H+) which has atomic mass 1.0072766.)
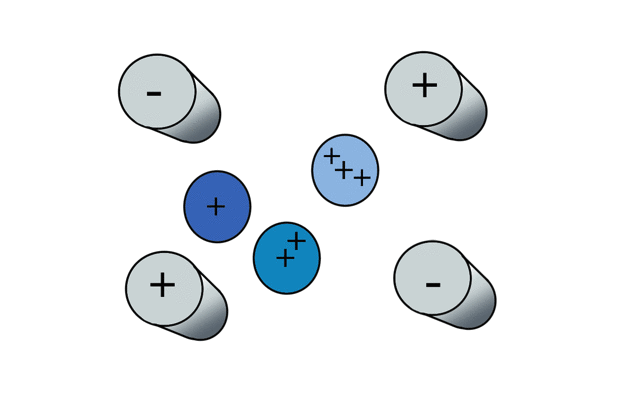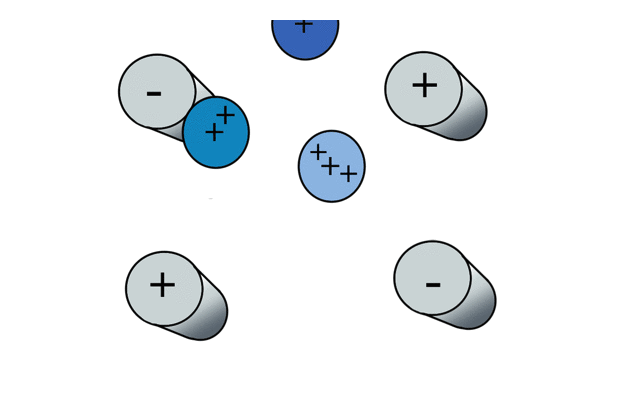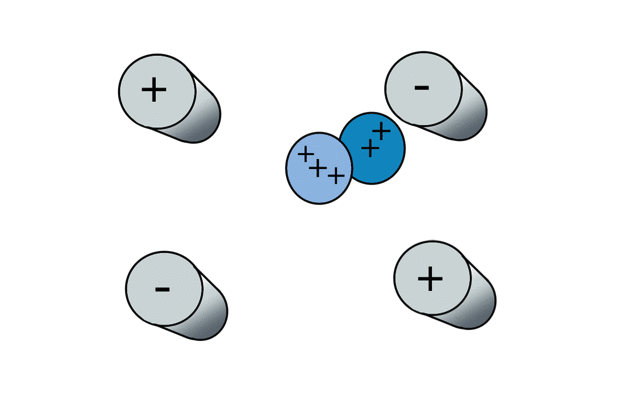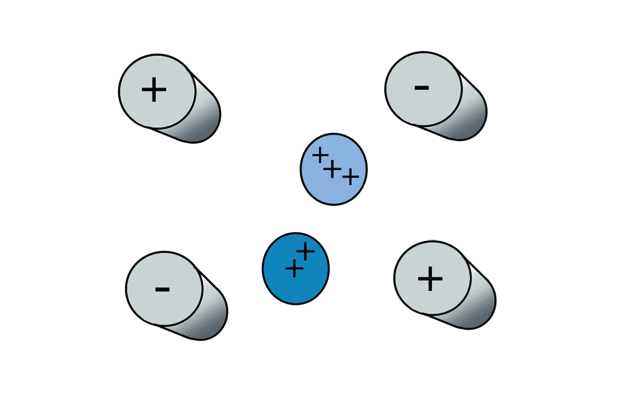
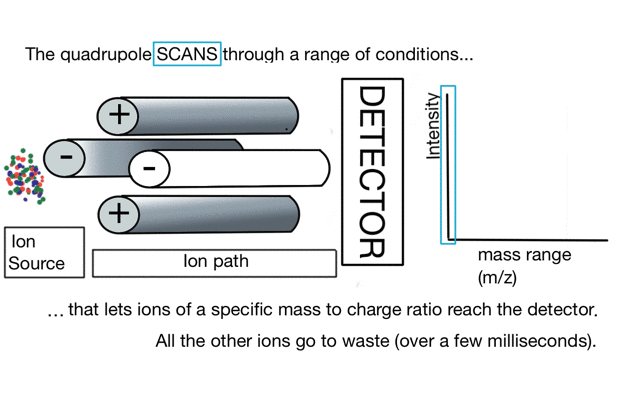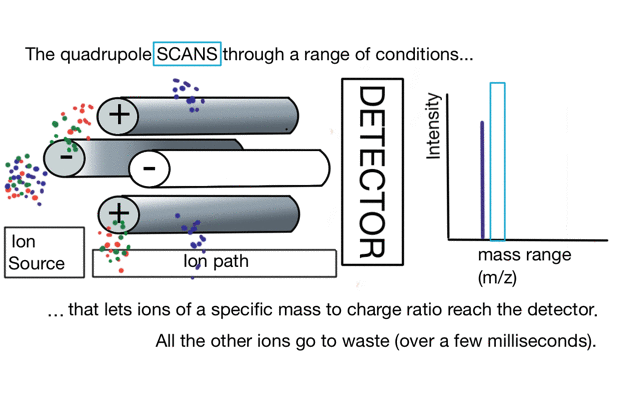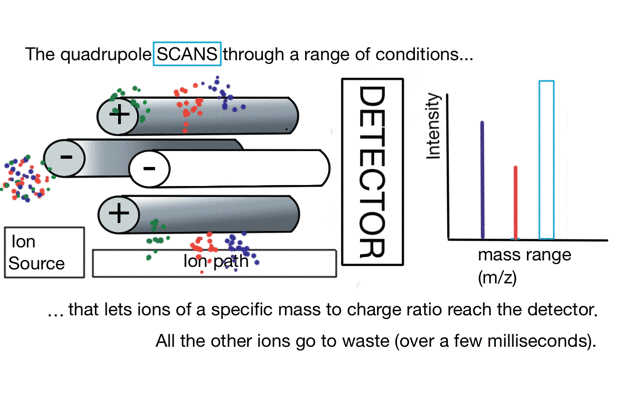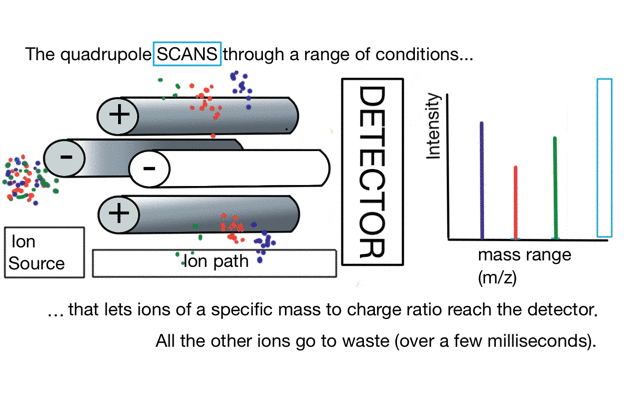
In the cartoon below of a quadrupole mass spectrometer, we have a source of red and blue ions on the left hand side and set the condition of the quadrupole to "red". Only the red ion has a stable path to the detector.

If we change the quad conditions to "blue" then only the blue ion makes it to the detector.
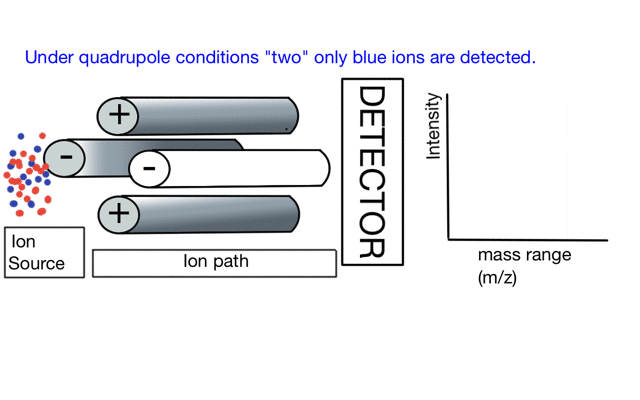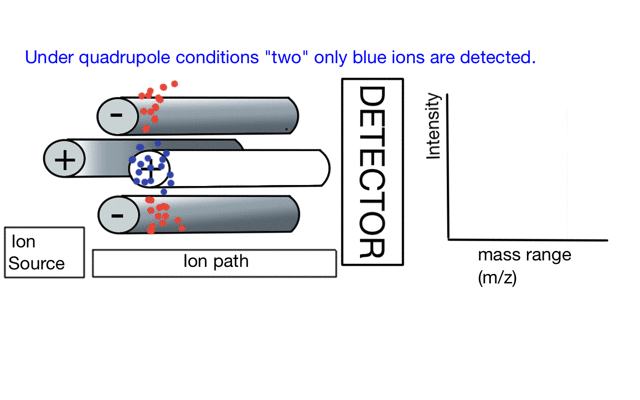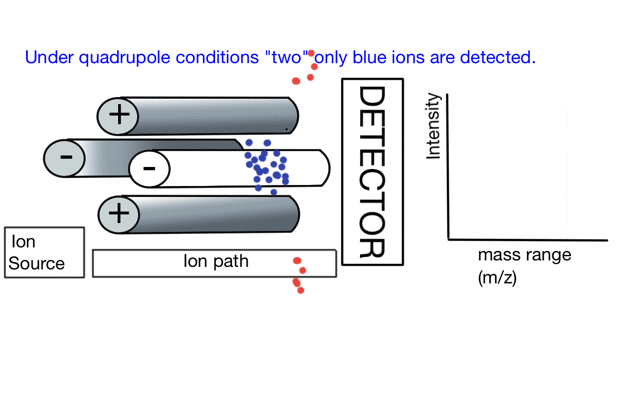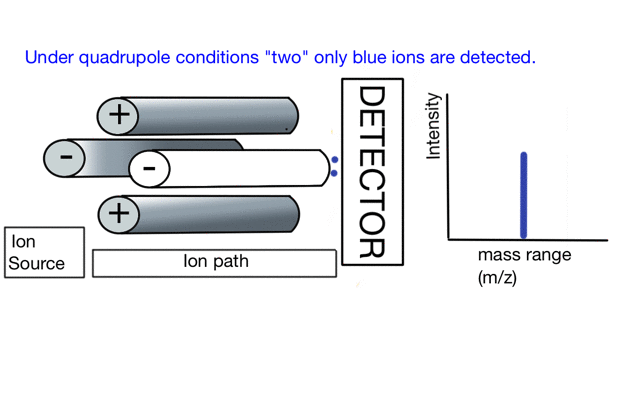
So to build a spectrum over a mass range we have a constant supply of ions from the left hand side and sequentially change the RF amplitude of the quadrupole to allow different ions to reach the detector. In other words, the quad scans a mass range to the detector to build a mass spectrum.
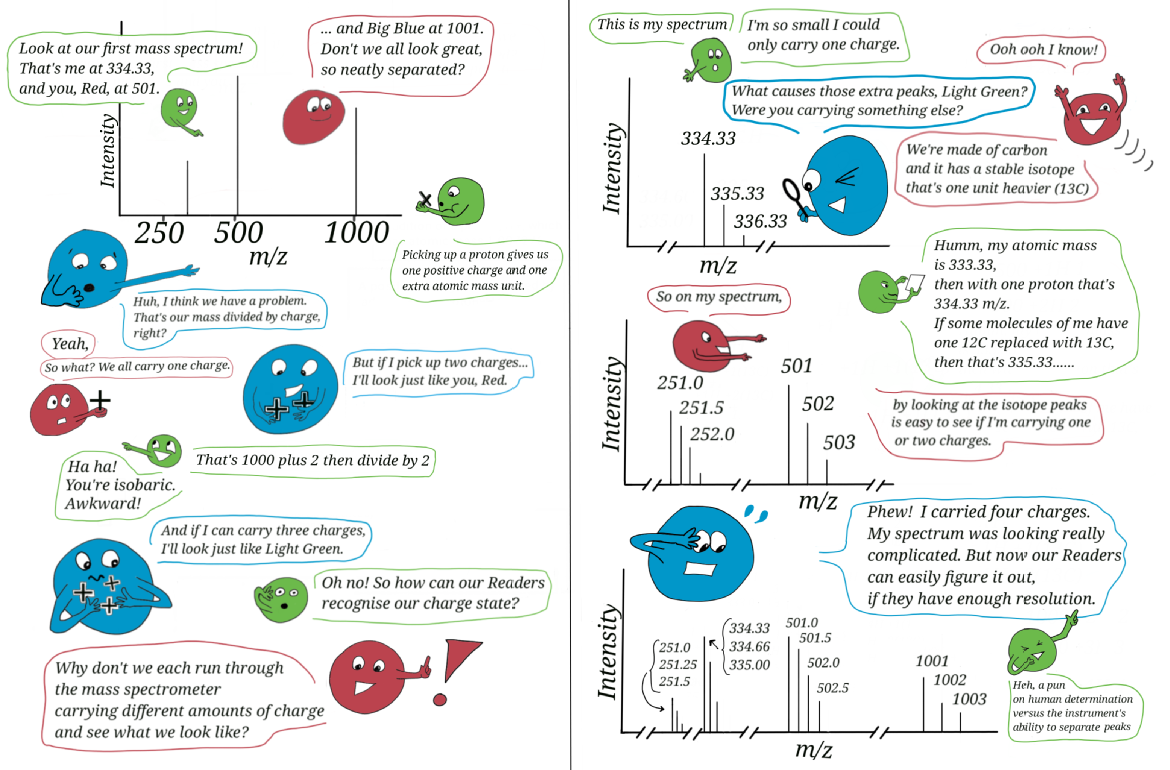
The importance of carbon isotopes for determining charge state of peptides
It is important to understand how to determine the charge state of peptides, I usually offer an example a bit like the cartoon below. This can provide a nice way to lead discussion to monoisotopic mass and how isotope distributions will vary with composition. For example, consider an average peptide composition versus compounds that are rich in nitrogen or sulphur, or consider if large large molecules, like proteins, really exist in a monoisotopic form. Of course, if dealing with a simple mixture (say 1000 in four charge states) you might be able to recognise the charge state from the distribution of main peaks without zooming in on the isotopes.
Electrospray ionisation
Gentle ionisation is essential for large molecules (like peptides). We often prepare peptides in solution so we need to move them from liquid to the gas phase for mass spectrometry. Without ionisation liquids and mass spectrometers are not compatible.
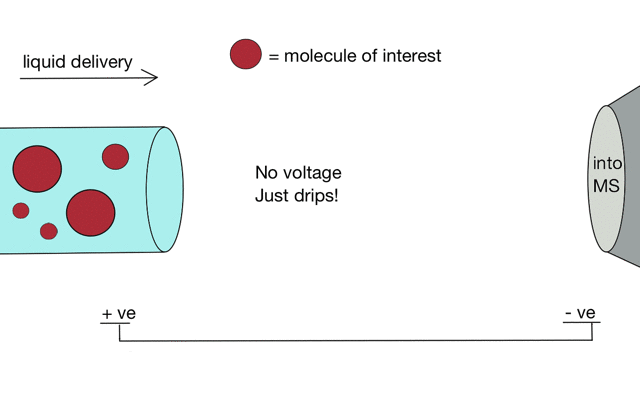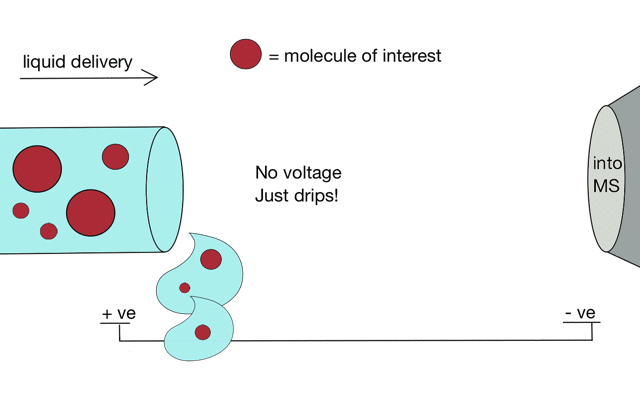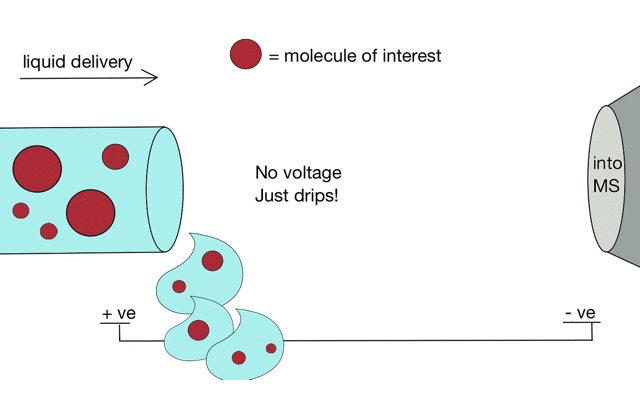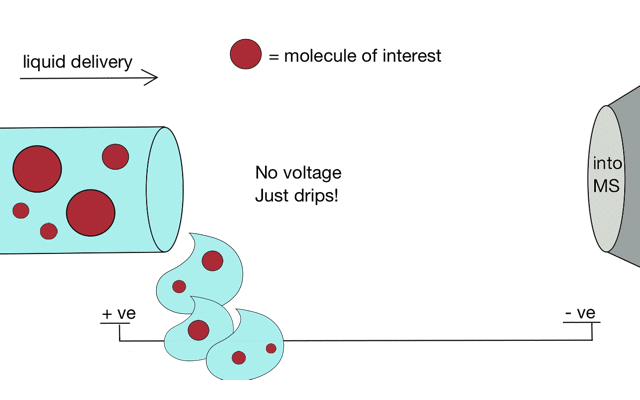
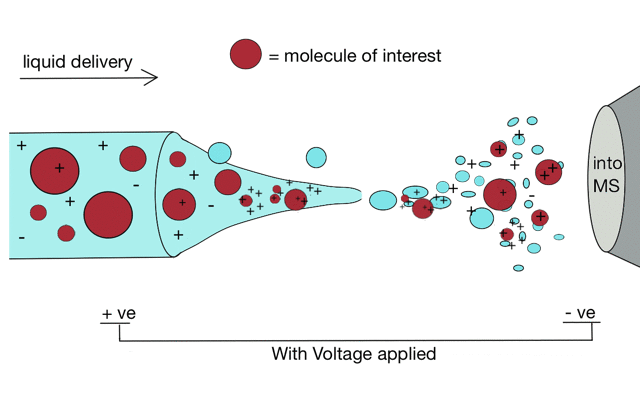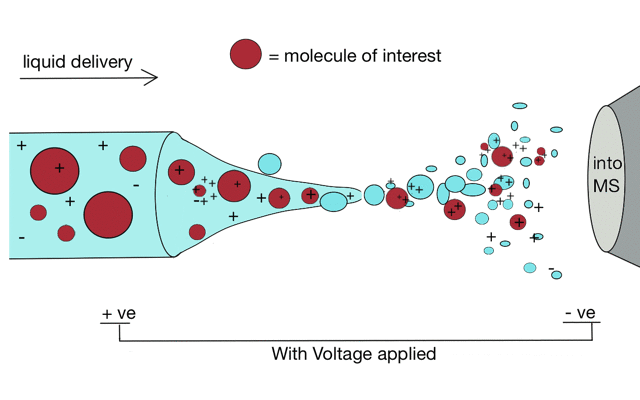
But if we apply some voltage, in this case to a positively charged solution (say peptides in acid) then the positively charged material is drawn towards the mass spectrometer. If the solvent (light blue) evaporates as the drop forms then the positive charges become more crowded, until the repulsion between lots of positively charged ions overcomes the surface tension of the droplet. Then - boom - the droplet explodes creating smaller droplets and gaseous ions. This process repeats until you end up with positive ions in the gas phase.
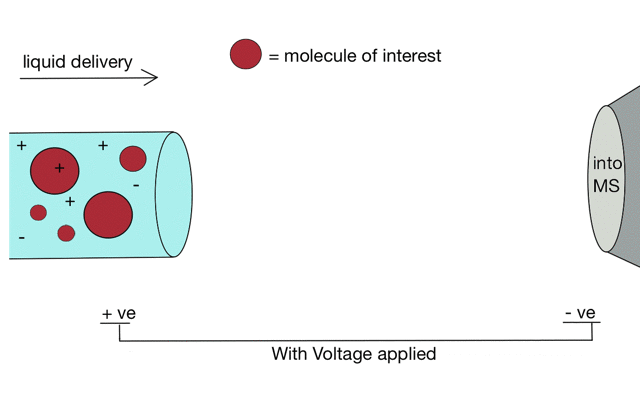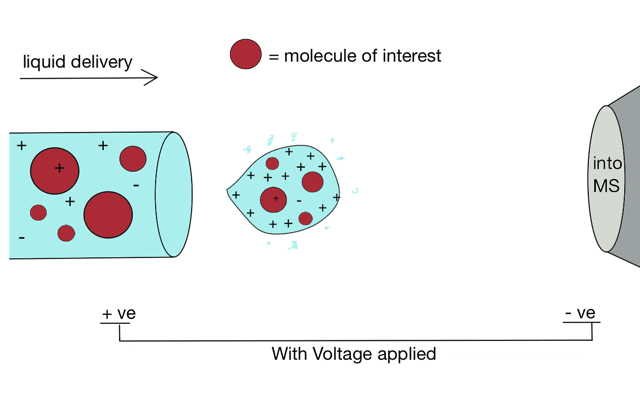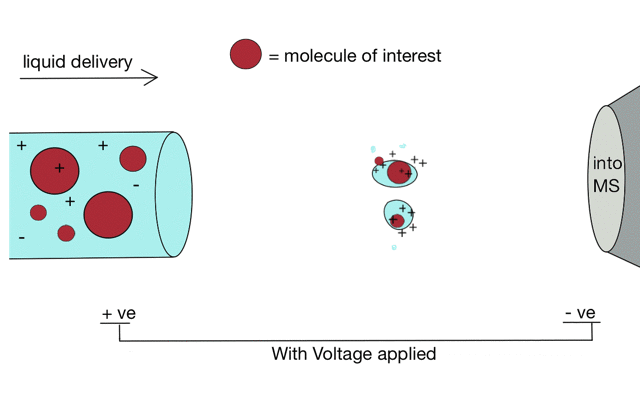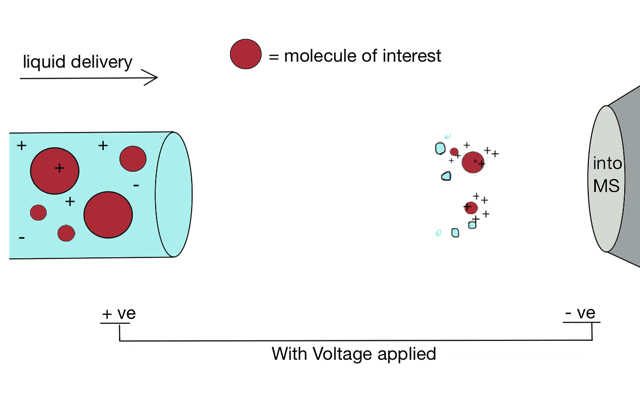
With a continuous supply of liquid, say from a chromatography system, something more like a cone forms.
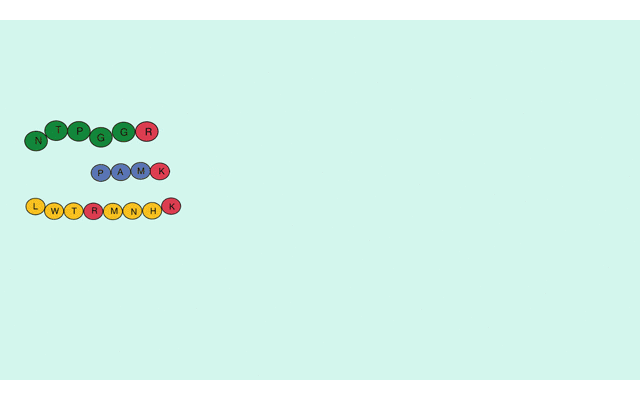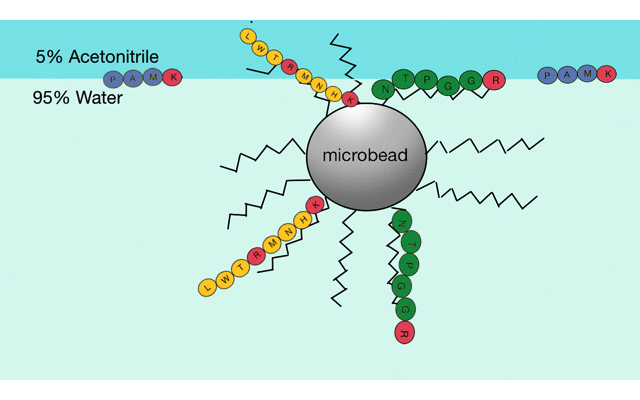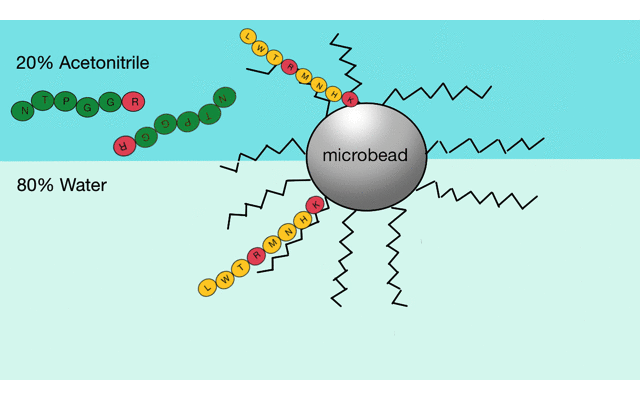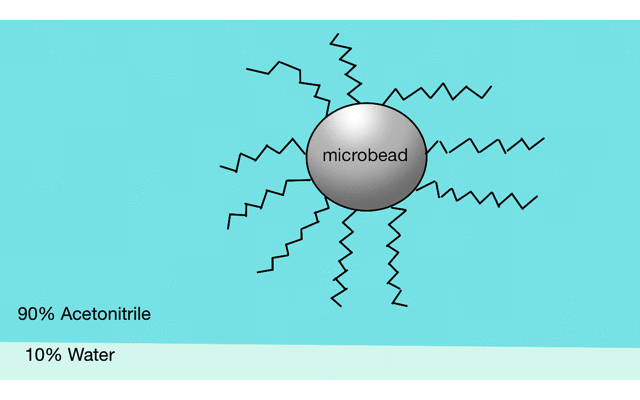
Reverse phase liquid chromatography
School of Life Sciences,
University of Warwick
I used to lead our Proteomics Research Technology Platform
Note for animators
I use Autodesk Sketchbook Pro (flipbook) to make the images and then PicGif, because I use around 200 ms per frame (not a typical 12 or 24 fps that flipbook expects). A Wacom tablet helps too.
Useful links
BSPR British Society for Proteome Research
BMSS British Mass Spectrometry Society
ASMS American Society for Mass Spectrometry
Mass Spec Pro has lots of more advanced tutorials under 'Technology"
