News Library
Nanodiamonds bring back sparkle to cleaning
Nanodiamonds have been found to help loosen crystallized fat from surfaces in a project led by Dr Andrew Marsh at University of Warwick. The tiny carbon particles transform the ability of surfactants to shift dirt in cold water, findings that could bring eco friendly low temperature laundry cycles.
The research is published in ACS Applied Materials and Interfaces and highlighted in the Daily Mail and Daily Telegraph, 26 June.
Nanodiamond Promotes Surfactant-Mediated Triglyceride Removal from a Hydrophobic Surface at or below Room Temperature Xianjin Cui, Xianping Liu, Andrew S. Tatton, Steven P. Brown, Haitao Ye, and Andrew Marsh ACS Applied Materials and Interfaces 2012, http://dx.doi.org/10.1021/am300560z
Unwin and O'Reilly win prestigious RSC awards
Pat Unwin and Rachel O’Reilly win 2012 Royal Society of Chemistry Awards in recognition of significant contributions to their research fields
Resolutely Pure Helices
Warwick antibiotic complexes appear in Nature Chemistry News & Views article by Janice Aldrich-Wright.
- "...a simplicity and an elegance of design that should be a source of inspiration for future studies...
- ...easily tuned to explore structure–function relationships that are crucial in biological applications...
- ...good antibiotic activity against...MRSA and E. coli as well as low toxicity to Caenorhabditis elegans.
- ...potential to develop into a family of cost-effective antibiotics."
Stimuli Responsive Polymers Tuned for Specific Intracellular Degradation
The Gibson group have reported in Chemical Commmunications on a new route to obtain polymers containing disulfide linkages in their backbone. These linkages are appealing for drug delivery applications as they are stable in the blood stream but can be specifically degraded inside cells. Traditional controlled radical polymerizations produce all-carbon backbones which do not degrade but, in this paper, the authors demonstrate how a 2-stage polymerization process can be used to incorporate disulfides. Furthmore this allows the use of functional monomers which result in 'smart' materials capable of responding to thermal gradients.
This was published in Chemical Communications link
More information on the Gibson Group can be found here link
‘Left-handed iron corkscrews’ point the way to new weapon in battle against superbugs like MRSA

 Scientists at the University of Warwick have taken inspiration from corkscrew structures found in nature to develop a new weapon in the fight against infections like E-coli and MRSA.
Scientists at the University of Warwick have taken inspiration from corkscrew structures found in nature to develop a new weapon in the fight against infections like E-coli and MRSA.
Researchers have created a new synthetic class of helix-shaped molecules which they believe could be a key tool in the worldwide battle against antibiotic resistance. By twisting molecules around iron atoms they have created what they term ‘flexicates’ which are active against MRSA and E-coli - but which also appear to have low toxicity , reducing the potential for side effects if used in treatment. The work is published in Nature Chemistry.
The new structures harness the phenomenon of ‘chirality’ or ‘handedness’ whereby the corkscrew molecules could be left-handed or right-handed. By making the most effective ‘hand’ to attack a specific disease, the University of Warwick research paves the way towards a more targeted approach to killing pathogens. In the case of E-coli and MRSA, it is the left ‘hand’ which is most effective.
Professor Peter Scott of the University of Warwick’s chemistry department said although this particular study concentrated on flexicates’ activity against MRSA and E-coli, the new method of assembly could also result in new treatments for other diseases.
“It’s a whole new area of chemistry that really opens up the landscape to other practical uses. These new molecules are synthetically flexible, which means that with a bit of tweaking they can be put to use against a whole host of different diseases, not just bugs like MRSA which are rapidly developing resistance to traditional antibiotics. Flexicates are also easier to make and produce less waste than many current antibiotics.”
Scientists have long been able to copy nature’s corkscrew-shaped molecules in man-made structures known as helicates – but they have thus far not been able to use them in fighting diseases. One of the key issues is the problem of handedness. Sometimes ‘left-handed’ molecules in drugs are the most effective at combating some disease, while sometimes the ‘right-handed’ version works best. Until now, scientists working with helicates have found it difficult to make samples containing just one type of corkscrew; either the right- or left-handed twist.
With flexicates, the University of Warwick scientists have succeeded in making samples containing just one type of twist – resulting in a more targeted approach which would allow the drug dosage to be halved. Flexicates solve other problems encountered by helicates, as they are easier to optimise for specific purposes, are better absorbed by the body and are easier to mass-produce synthetically.
Professor Scott said: “Drugs often have this property of handedness - their molecules can exist in both right and left handed versions but the body prefers to use only one of them. For this reason, drug companies have to go to the trouble of making many traditional molecules as one hand only. What we have done is solve the ‘handedness’ problem for this new type of drug molecule. By getting the correct hand we can halve the drug dose, which has the benefits of minimising side effects and reducing waste. For patients, it’s safer to swallow half the amount of a drug. Our work means that we can now make whichever hand of the corkscrew we want, depending on the job we require it to do.”
Notes to editors
The study, entitled Optically pure, water-stable metallo-helical ‘flexicate’ assemblies with antibiotic activity, is published in Nature Chemistry.
The research was also supported financially by EPSRC.
It is authored by Peter Scott, Suzanne Howson, Guy Clarkson and Alison Rodger from the University of Warwick, Albert Bolhuis from the University of Bath and Viktor Brabec and Jaroslav Malina from the Academy of Sciences of the Czech Republic.
When the paper is published it can be retrieved at http://dx.doi.org/10.1038/NCHEM.1206
Contact details
Professor Peter Scott is available on +44 (0) 24 7652 3238 or peter.scott@warwick.ac.uk
University of Warwick press officer Anna Blackaby is available on + 44 (0) 2476 575910 or + 44 (0)7785 433155 or a.blackaby@warwick.ac.uk
Front Page Artwork by Elisabeth Heissler http://ehgraphicdesign.co.uk/
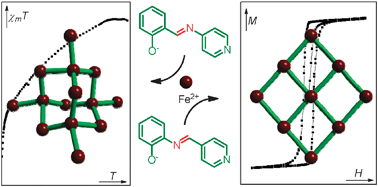
Simple compounds, hard magnets
New magnetic materials have been created from simple components by Lihong Li, a Warwick Postgraduate Research Fellow and co-workers in the Scott group at Warwick University. The resarchers have engineered magnetic diamond-like 3D networks and layered 2D net structures. A rare example of a molecular hard magnet (which like a regular magnet keeps its magnetic properties over time) is produced. The chemistry is simple, reliable and scalable so the group hope to make many new materials in the future for applications in data storage, and quantum computing. The work is published in Chemical Communications.
Precious metal materials
Walton’s group, in collaboration with colleagues in the Department of Physics and at Johnson Matthey plc have a paper published this week in the RSC journal Chemical Science: this describes mild synthetic chemical routes to complex extended structures that contain the metal iridium in various oxidation states. This illustrates the scope for the discovery of new functional materials by exploration of novel reaction conditions and using the chemistry of lesser studied elements.
http://pubs.rsc.org/en/content/articlelanding/2011/sc/c1sc00192b/
Tweakable chiral magnetic materials
Magnetic interactions between metal atoms in a family of chiral materials respond to subtle changes in their organic chemistry. The work forms part of the PhD research by Lihong Li in the Scott group in Warwick Chemistry department. Read the article at Inorg. Chem. 2011
Corkscrew Conductors
A new family of semiconductors in which the electricity is transported through nanoscopic helices have been reported by the Scott group at Warwick. The work forms part of the PhD research by former Warwick Postgraduate Research Fellow
 , Dr Nikola Chmel
, Dr Nikola Chmel
 . Read the paper at Inorg. Chem. 2011
. Read the paper at Inorg. Chem. 2011
Graphene oxide’s solubility disappears in the wash
|
Drs Rourke and Wilson’s team made their discovery when treating the graphene oxide with sodium hydroxide (NaOH) in an attempt to increase the usefulness of the oxygen containing functional groups believed to be bound to the graphene. Unfortunately it seemed to make things worse rather than better. Indeed at high enough concentrations of NaOH Dr Rourke was left with a black suspension. The Warwick led researchers recalled that it had been shown that oxidation debris adheres to carbon nanotubes but the weak nature of the connection of this oxidation debris to the carbon nanotubes meant that a wash with a base can simply remove the oxidative debris. Experiments showed that in that particular case oxidative debris was found to make up almost a quarter of the mass of the “oxidized carbon nanotubes”. The researchers felt a similar process maybe happening in the Graphene Oxide they were studying. The results may also help explain the inordinately high levels of oxygen people were claiming to find in graphene oxide. Chemists were already struggling to identify enough plausible carbon to oxygen bonds to accommodate the amounts of oxygen believed to form part of graphene oxide.
The remaining liquid was also dried to give a white powder that the Warwick researchers showed contained the “oxidative debris” or OD; the OD was shown to be made up exclusively of small, low molecular weight compounds (i.e. less than 100 atoms). The graphene oxide recovered from washing process formed about 64% of the mass of the “graphene oxide” at the start of the process. The recovered OD or oxidative debris formed at least 30% of the weight of the mass of the original “graphene oxide”. Drs Rourke and Wilson’s team believe this shows that much of the oxygen that was believed to be closely bonded to the carbon in the graphene oxide was actually not bonded at all but simply lying on top of the graphene sheets, loosely connected to them as “oxidative debris”. This oxidative debris contained a large quantity of oxygen that simply came out in the wash when the graphene oxide was treated with sodium hydroxide. This creates a significant proble Drs Rourke and Wilson say “Our results suggest that models for the structure of graphene oxide need revisiting. These results have important implications for the synthesis and application of chemically modified graphene particularly where direct covalent functionalization of the graphene lattice is required.” The paper entitled: The Real Graphene Oxide Revealed: Stripping the Oxidative Debris from the Graphene-like Sheets by Dr. Jonathan P. Rourke, Priyanka A. Pandey, Joseph J. Moore, Matthew Bates, Neil R Wilson (all of the University of Warwick), and Dr Ian A. Kinloch, Prof. Robert J. Young (The University of Manchester), has just been published in Angewandte Chemie DOI: 10.1002/anie.201007520. Notes for editors: The researchers thank Dave Hammond for help with thermogravimetric analysis (TGA), Lijiang Song for help with mass spectrometry, and Ajay Shukla for help with X-ray photoelectron spectroscopy (XPS), the Midlands Physics Alliance Graduate School for a scholarship. The TEM, TGA, and XPS instruments as well as the mass spectrometer used in this research were purchased with support from Advantage West Midlands (part funded by the European Regional Development Fund) as part of the Science City programme. For further information please contact: Dr Jonathan P. Rourke Peter Dunn, Head of Communications PR23 8th March 2011 |
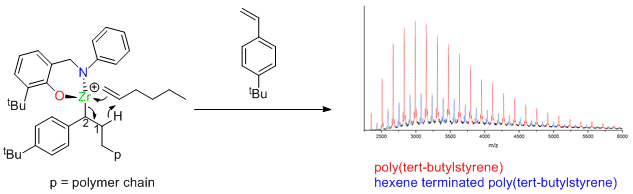
Zirconium catalysts not dead, just resting.
Dormant polymerization catalysts are given a rude awakening by Giles Theaker, Peter Scott and Warwick Chemistry Alumnus Colin Morton at Infineum. The results indicate how many old catalysts, thought to be dead, are just in a dormant state. The results published in the American Chemical Society journal Macromolecules describe a new mechanism for polymerization of styrenes.
Scott Group detects chiral building blocks for new materials
Pairs of building blocks are shown using spectroscopic and electrochemical techniques to associate in solution before forming new types of charge transfer material relevant to the search for chiral conductors. The work forms part of the PhD research by former Warwick Postgraduate Research Fellow , Dr Nikola Chmel
, Dr Nikola Chmel .
.
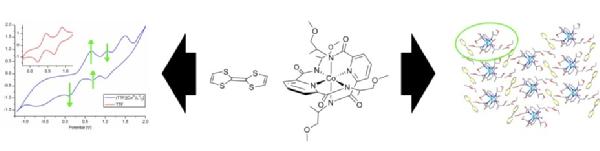
read the paper: http://dx.doi.org/10.1039/C0DT01184C