Bacterial Metallothioneins
Metallothioneins are small, cysteine-rich proteins with an unparalleled capacity to bind metal ions. They occur in species of all eukaryotic kingdoms (see Overview), as well as in various bacteria (see link to book chapter on bacterial MTs and a recent review: Bacterial metallothioneins: past, present, and questions for the future
and a recent review: Bacterial metallothioneins: past, present, and questions for the future ).
).
Metallothioneins, together with other proteins, are thought to take care of metal ions inside cells. The simplified schematic below illustrates how we think copper and zinc are handled by bacterial cells.

Although much progress has been made in the case of copper, the intracellular fate of zinc is still a matter of speculation - surprisingly, if one considers that hundreds of enzymes require zinc for their function, and that the classical zinc finger is the most abundant protein domain found in the human genome. It is still not known how all these proteins acquire their zinc cofactor.
In higher organisms, the picture is more complicated yet.
.
.
.
We have determined the first 3D structure of a bacterial metallothionein, SmtA from the cyanobacterium Synechococcus PCC7942.
The picture illustrates how metallothioneins envelop small portions of "inorganic" metal-sulfur clusters. The big yellow balls are sulfur atoms from cysteine, which almost obscure the purple balls of zinc. The two blue balls are nitrogens from His imidazole side-chains. The latter were the first histidines demonstrated to be zinc-binding in a metallothionein. Previously, this was perceived as an exotic exception, but inspection of sequences for non-vertebrate metallothioneins (i.e. the majority of all metallothioneins), reveals that this is not quite that exceptional (see page on "histidines in metallothioneins"). Our more recent work focuses on several metallothioneins from plants (see Plant MTs) and invertebrate animals (see Metallothioneins from worms), and in several cases, we have shown that the histidine residues play absolutely pivotal roles in those proteins.
of "inorganic" metal-sulfur clusters. The big yellow balls are sulfur atoms from cysteine, which almost obscure the purple balls of zinc. The two blue balls are nitrogens from His imidazole side-chains. The latter were the first histidines demonstrated to be zinc-binding in a metallothionein. Previously, this was perceived as an exotic exception, but inspection of sequences for non-vertebrate metallothioneins (i.e. the majority of all metallothioneins), reveals that this is not quite that exceptional (see page on "histidines in metallothioneins"). Our more recent work focuses on several metallothioneins from plants (see Plant MTs) and invertebrate animals (see Metallothioneins from worms), and in several cases, we have shown that the histidine residues play absolutely pivotal roles in those proteins.
SmtA turned out to be a rather special protein also in other respects. In contrast to previously characterised MTs, it contains well-defined elements of secondary structure (a β bridge, a β hairpin, and a short α helix). We found that this fold is very similar to a recently defined new zinc finger fold, called the “treble-clef” (like that in sheet music) fold, although sequence comparisons or even advanced fold recognition tools fail to detect this similarity.
The insight into which residues are vital for the SmtA fold has, in combination with BLAST searches, enabled the identification of homologous and structurally related proteins from other bacteria. We have confirmed by 2D NMR that representative proteins from Pseudomonads and E. coli adopt the same fold as SmtA, and have started to characterise the metal-binding
properties of these new proteins.

SmtA has another unique feature – it contains an inert zinc ion, that does not exchange with external Cd(II) or Zn(II). This is unheard of for either metallothioneins or zinc fingers. In the case of zinc exchange, we have developed (in collaboaration with the group of Prof. Patrick Langridge-Smith, University of Edinburgh) a new approach using stable zinc isotopes and the ultra-high resolution power of Fourier-Transform Ion-Cyclotron-Resonance Mass Spectrometry. Reacting Zn4SmtA with an excess of 67ZnCl2 for more than 100 hours resulted in the exchange of only three of the four bound zinc ions.
The top FTICR mass spectrum shows the isotopic distribution of natural abundance Zn4SmtA. Zinc has isotopes of masses 64, 66, 67, 68, and 70. 67Zn has a natural abundance of only 4.1%. The main isotope is 64Zn with 48.6%.
The middle spectrum refers to 67Zn4SmtA, which had been generated by reconstituting the apo-protein with 67Zn2+. The mass envelope is much narrower due to the absence of other zinc isotopes.
The bottom spectrum refers to a natural abundance sample that had been reacted for 99h at 310K with a 10-fold (with respect to zinc) excess of 67Zn2+. The modelled intensities are consisten with the incorporation of 2.75 67Zn; this is close to the expected equilibrium value of 2.7, for exchange of 3 ions.
We hypothesise that metallothioneins have the capability in vivo to release metal ions to suitable acceptors. We have probed zinc release in vitro using the metal chelator EDTA, and have followed the reaction by ESI-MS, NMR, and UV-Vis spectroscopy, and also employed site-directed mutagenesis to probe the role of the histidine residues in the metal release reaction.
The zinc ions in the SmtA cluster have different release rates when reacting with external metal binding agents such as EDTA. One site reacts much slower than the other three. Mutation of His40 to a Cys residue generated a much more reactive protein, but with one site still reacting more slowly than the others. Crucially, using an oxidised version of the His49Cys mutant, we were able to show that the reaction proceeds via site C only, as blocking of this site results in a stable three-zinc cluster. The scheme below summarises what we know about the reaction mechanism.
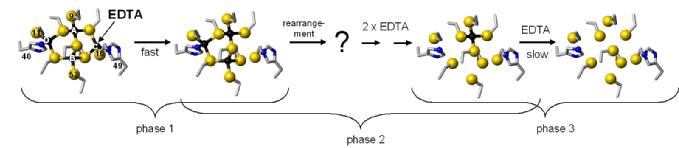
Intriguingly, site C is the most variable site in homologous bacterial metallothioneins. We hypothesise that this site is used to "tune" the metal transfer reactions of bacterial MTs. We are currently working on several homologues of SmtA, in order to understand how thermodynamics and kinetics of metal binding are correlated to protein structure.
