Plasma metalloproteins
One of the many compartments in biological systems which are involved in metal ion homeostasis is the blood plasma of vertebrates.
It is for example known that the zinc concentration in human blood is ca. 10-20 micromolar. The largest part (about three quarters) of plasma Zn(II) is bound by human serum albumin, which is present at a concentration of ca. 600 micromolar - it is thus the most abundant protein in plasma. Besides its likely, but not well understood role in zinc homeostasis, albumin performs many functions; in particular, it is resposible for the transport of fatty acids, bilirubin, and many xenobiotics including drugs.
Together with Dr. Alan Stewart and Prof. Peter Sadler, we have previously discovered the location of the high-affinity zinc-binding site on human albumin (see: Interdomain zinc site on human albuminLink opens in a new window). Intriguingly, the site lies at the interface between two domains:
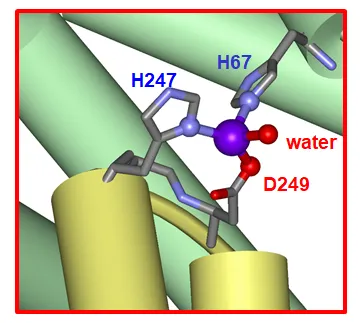
The image shows the structure of the high-affinity zinc site on human albumin, as determined by X-ray crystallography (Circulatory zinc transport is controlled by distinct interdomain sites on mammalian albumins)Link opens in a new window. Domain I is shown in green, and domain II in yellow, the zinc zinc ion is shown in purple. Besides two histidine residues, an aspartate and an external ligand such as a water coordinate the zinc ion.
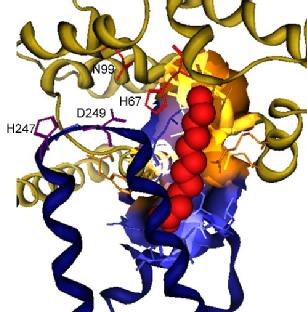
The major zinc site is adjacent to one of several binding sites for fatty acids, and we have hypothesised that the binding of fatty acids and zinc to its major site may be mutually exclusive. This potential allosteric mechanism may mediate zinc signals in the blood.
This has been verified experimentally (in vitro): A Molecular Mechanism for Modulating Plasma Zn Speciation by Fatty AcidsLink opens in a new window. Possible downstream effects are explored together with Dr. Alan Stewart (University of St Andrews) in Plasma free fatty acid levels influence Zn2+-dependent histidine-rich glycoprotein–heparin interactions via an allosteric switch on serum albumin.Link opens in a new window
Allosteric binding of metals and fatty acids is also the molecular mechanism that underlies a diagnostic test for early detection of heart attacks: Allosteric Inhibition of Cobalt Binding to Albumin by Fatty Acids: Implications for the Detection of Myocardial Ischemia. Link opens in a new window
More recently, we have deomstrated that the decreased zinc affinity of fatty-acid loaded albumin alters the speciation of zinc in plasma, with a redistribution of zinc to higher-molecular-weight proteins when fatty acid levels are high:
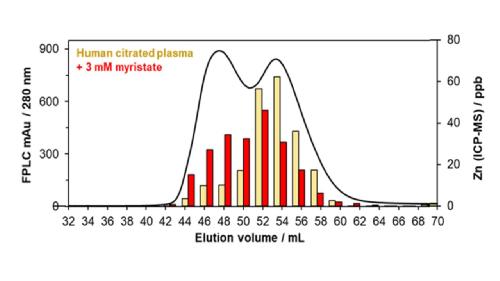
The image shows a metalloproteomics experiment. This involved size-exclusion chromatography of human plasma. The black line refers to protein (absorption at 280 nm), the red and yellow bars refer to Zn levels in chromatographic fractions, with (red) or without (yellow) added fatty acid (myristate, C14:0).
The alteration in speciation may also increase the uptake of Zn2+ into cells:
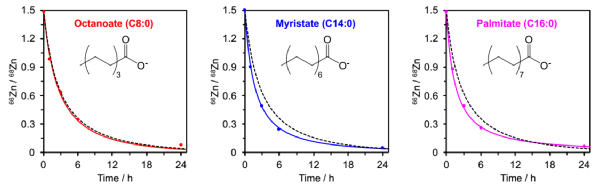
For these experiments, endothelial cells were incubated with mixtures of bovine serum albumin, isotopically pure 68Zn2+, with and without fatty acids. The plots show the changes in 66Zn/68Zn isotopic ratios within the cells over time in absence (black broken line) and presence (coloured lines) of different fatty acids. Octanoate is known to be too short to elicit the allosteric swithc and does not impar zinc binding. (Albumin-mediated extracellular zinc speciation drives cellular zinc uptake, Chem. Commun. 2022).
The crosstalk between zinc and fatty acids may affect processes in plasma - including blood clotting (The Interplay between Non-Esterified Fatty Acids and Plasma Zinc and Its Influence on Thrombotic Risk in Obesity and Type 2 Diabetes (2021))Link opens in a new window and insulin dynamics (Fatty acids may influence insulin dynamics through modulation of albumin-Zn2+ interactions (2021).
Further work, in collaboration with Dr. Alan Stewart (University of St Andrews) and Profs. Marco Zezzi-Arruda and Carlos Ramos, Campinas, Brazil, is ongoing.
For further reading, see our reviews in Biochemical Society TransactionsLink opens in a new window, BBA, and Current Trends in Medicinal Chemistry, Link opens in a new windowand the two papers Structure, Properties, and Engineering of the Major Zinc Binding Site on Human AlbuminLink opens in a new window and Plasma fatty acid levels may regulate the Zn2+-dependent activities of histidine-rich glycoprotein. Link opens in a new window
Also see:
