Publications
A complete up-to-date list of publications of Robert can be found on Google Scholar and full-texts might be available from the Warwick Research Active Portal (WRAP).
In Situ Lipid Interactions of an Anticancer Metal Complex
 |
Ed Lant's paper on cool molecular helicopters that also fluoresc entitled: "In Situ Lipid Interactions of an Anticancer Metal Complex". A big collaboration with Peter Sadler's lab and collaborators from Diamond (Maria Harkiolaki) and others. In this paper, Ed uses an integrated multimodal imaging workflow of cryogenic super-resolution fluorescence microscopy and soft X-ray tomography, Orbitrap secondary ion mass spectrometry, and inductively coupled mass spectrometry to reveal unexpected targeting of a half-sandwich cyclopentadienyl Rh(III) phenylazopyridine anticancer complex to cellular lipid membranes and lipid droplets. The complex accumulates in plasma membranes with a surprisingly intense switch-on luminescence in living cancer cells, drives remodelling of lipid droplet architecture, and penetrates deeply into lipid-rich tissue environments. DFT modelling shows strong supramolecular interactions between the complex and glycerophosphorylcholine lipids. |
How to safeguard against the low hanging fruit being rotten?
 |
Recent clinical results suggest that early immunotherapy is more efficacious than late immunotherapy. In a commentary lead by Seline Ismail-Sutton, we argue that "Personalising chronotherapy of immune checkpoint blockade" is important. Chronotype as a factor should be looked at in pragmatic chrono-immunotherapy trials. |
Cationic antimicrobial copolymers reveal immunomodulatory properties in LPS stimulated macrophages in vitro
 |
Now out as paper in Biomacromolecules. Led by Sophie Laroque from the Perrier Lab in Warwick and in collaboration with Katherine Locock at CSIRO Australia, we show the anti-inflammatory effects of novel cationic co-polymers that also show anti-microbial activity. Win-win for sepsis patients. |
Cyclic Peptide – Polymer Conjugate Nanotubes for Delivery of SN-38 in Treatment of Colorectal Cancer Model
New paper by Sophie Hill reporting on her drug delivery work during her PhD in the Perrier lab.
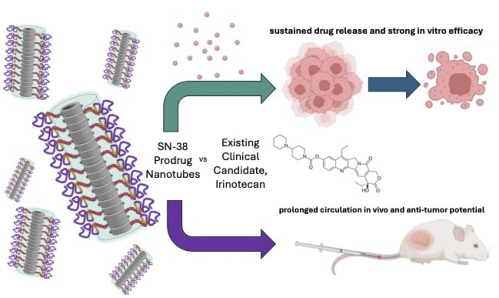 |
Cyclic peptide-polymer conjugate nanotubes have been shown to be powerful drug delivery vectors, due to their propensity for dynamic self-assembly, high aspect ratio morphology and structural interchangeability. Building upon previous studies that demonstrate the shielding abilities of the polymeric corona of nanotubes to enhance pro-drug bond stabilities and modulate hydrolysis, we utilise here the concept of a hydrophobic core building block with multiple drug units to improve drug loading capacity and overall efficiency of the nanotube carriers. By leveraging the intermolecular features of the drug core to strengthen assembly, we hypothesise that these nanotubes have the potential as a responsive supramolecular delivery system whereby upon full hydrolysis of the labile drug, these core forming interactions disappear, and nanotubes can fall apart and undergo clearance. Herein, we explore the self-assembly, in vitro efficacy and in vivo pharmacokinetic and anti-tumour pharmacodynamics of these nanotubes in colorectal cancer models, comparing the potent topoisomerase inhibitor SN-38 with its clinically-used parent pro-drug irinotecan. |
Machine learning assisted classification of cell and brain penetrating peptides
Great collaboration with Seb Perrier and Gabriele Sosso from Warwick Chemistry. Vito's first paper from his PhD work in the IBR MRCDTP.
 |
Crossing the blood-brain barrier (BBB) remains a major obstacle for central nervous system therapeutics. Short peptides have emerged as promising vectors, including cell-penetrating peptides (CPPs) and brain-penetrating peptides (BPPs). However, the structural and physicochemical features that distinguish CPPs from BPPs remain poorly understood, limiting rational design. Here, we systematically analysed their amino acid composition, sequence distribution, and physicochemical descriptors. Our findings suggest that BBB penetration is not a simple extension of cell penetration but requires finely tuned physicochemical properties. This study provides mechanistic insights into BPP design and highlights machine learning as a valuable tool for engineering next-generation BBB-penetrating peptides and peptide-mimetic materials. |
