Investigator Site File Documentation
On this page you will find all relevant documents and resources for setting up and conducting the trial at site.
To help you with this, please find a version control log listing all of our documents here.
If you wish to print these documents to create a paper ISF, please find an index here. An ISF receipt can be found here.
To request previous document versions please email: stress-l@warwick.ac.uk
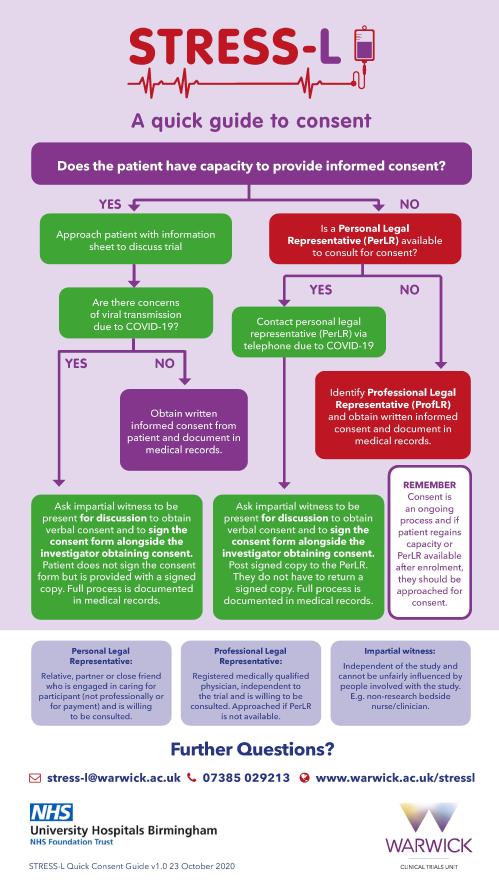
2 **NEW** Infographic tools are now available to assist with eligibility clarification and consent post COVID.

1. Reference Information
2. Protocol
3. Information for Participants
4. HRA
5. Regulatory
6. Individual Site Information and Approvals
7. Study Drugs
8. Laboratory
9. Monitoring
10. Data Collection
11. Safety Information
12. Trial Specific Working Instructions
1. Reference Information
Trial contacts and coordination details
2. Protocol
3. Information for Participants
Legal Representative Information Sheet
Short Patient Information Sheet
Legal representative consent form
4. HRA
5. Regulatory
6. Individual Site Information
Eligibility criteria pocket cards
**NEW** Eligibility Clarification
Landiolol infusion protocol compliance
Poster for clinical staff areas
Training for ICU clinical staff presentation
Training for non-GCP delegated clinical staff presentation
**NEW** Quick Guide to Consent
7. Study Drugs
Summary of product characteristics
8. Laboratory
Aide memoir sample schedule information
Labels for plasma storage boxes
9. Monitoring
10. Data Collection
Template: Participant screening log (excel)
Template: Participant screening log (word)
End of Trial Form and Sign-off statement
